Abstract
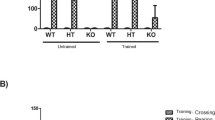
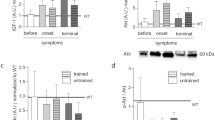
Duchenne muscular dystrophy (DMD) is a genetic disease associated with progressive skeletal muscle degeneration. In humans, DMD has an early onset, causes developmental delays, and is a devastating disease that drastically diminishes the quality of life of young individuals affected. The objective of this study was to evaluate the effects of a swimming protocol on memory and oxidative stress in an animal model of DMD. Male mdx and wild-type mice aged ≥ 28 days were used in this study. The animals were trained for a stepped swimming protocol for four consecutive weeks. The swimming protocol significantly reduced the levels of lipid peroxidation and protein carbonylation in the gastrocnemius, hippocampus, and striatum in the exercised animals. It also prevented lipid peroxidation in the diaphragm. Moreover, it increased the free thiol levels in the gastrocnemius, the diaphragm, and all central nervous system structures. The results showed that the protocol that applied swimming as a low-intensity aerobic exercise for 4 weeks prevented aversive memory and habituation in mdx mice.








Similar content being viewed by others
Data Availability
Data will be made available upon request.
References
Sinha R, Sarkar S, Khaitan T, Dutta S (2017) Duchenne muscular dystrophy: case report and review. J Fam Med Prim Care 6(3):654
Hoepers A, Alberti A, Freiberger V, Ventura L, Grigollo LR, Andreu CS et al (2020) Effect of aerobic physical exercise in an animal model of duchenne muscular dystrophy. J Mol Neurosci 70:1–13
Yucel N, Chang AC, Day JW, Rosenthal N, Blau HM (2018) Humanizing the mdx mouse model of DMD: the long and the short of it. npj Regen Med 3(1):1–11
Gevaerd MS, Domenech SC, Junior NGB, Higa DF, Lima-Silva AE (2020) Alterações fisiológicas e metabólicas em indivíduo com distrofia muscular de Duchenne durante tratamento fisioterapêutico: um estudo de caso. Fisiot Mov 23:93–103
Le Rumeur E (2015) Dystrophin and the two related genetic diseases, Duchenne and Becker muscular dystrophies. Bosn J Basic Med Sci 15:14–20
Alemdaroğlu Đ, Karaduman A, Yilmaz ÖT, Topaloğlu H (2015) Different types of upper extremity exercise training in duchenne muscular dystrophy: effects on functional performance, strength, endurance, and ambulation. Muscle & Nerve 51:697–705
Hyzewicz J, Ruegg UT, Takeda S (2015) Comparison of experimental protocols of physical exercise for mdx mice and duchenne muscular dystrophy patients. J Neuromuscul Dis 2:325–342
Aguirre LE, Villareal DT (2015) Physical exercise as therapy for frailty. Nestle Nutr Inst Workshop Ser 83:83–92
Jansen M, Alfen NV, Geurts ACH, Groot IJM (2013) Assisted bicycle training delays functional deterioration in boys with duchenne muscular dystrophy: the randomized controlled trial “No Use Is Disuse.” Neurorehabilit Neural Repair 27:816–827
Nikolai AL, Novotny BA, Bohnen CL, Schleis KM, Dalleck LC (2009) Cardiovascular and metabolic responses to water aerobics exercise in middle-aged and older adults. J Phys Act Health 6:333–338
Hyzewicz J, Tanihata J, Kuraoka M, Ito N, Miyagoe-Suzuki Y, Takeda S (2015) Low intensity training of mdx mice reduces carbonylation and increases expression levels of proteins involved in energy metabolism and muscle contraction. Free Radic Biol Med 82:122–136
Mazzardo-Martins L, Martins DF, Marcon R, Santos UD, Speckhann B, Gadotti VM et al (2010) High-intensity extended swimming exercise reduces pain-related behavior in mice: involvement of endogenous opioids and the serotonergic system. J Pain 11:1384–1393
Ishii H, Nishida Y (2013) Effect of lactate accumulation during exercise-induced muscle fatigue on the sensorimotor cortex. J Phys Ther Sci 25:1637–1642
Leussis MP, Bolivar VJ (2006) Habituation in rodents: a review of behavior, neurobiology, and genetics. Neurosc and Biobehav Rev 30:1045–1064
Ogihara CA, Schoorlemmer GH, Lazari MF, Giannocco G, Lopes OU, Colombari E, Sato MA (2014) Swimming exercise changes hemodynamic responses evoked by blockade of excitatory amino receptors in the rostral ventrolateral medulla in spontaneously hypertensive rats. BioMed Res Int 2014:1–9
Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79:583–593
Schubert MM, Washburn RA, Honas JJ, Lee J, Donnelly JE (2015) Exercise volume and aerobic fitness in young adults: the Midwest Exercise Trial-2. Springerplus 5:183
Gaesser GA, Poole DC (1996) The slow component of oxygen uptake kinetics in humans. Exerc Sport Sci Rev 24:35–71
Comim CM, Tuon L, Stertz L, Vainzof M, Kapczinski F (2009) Quevedo J (2009) Striatum brain-derived neurotrophic factor levels are decreased in dystrophin-deficient mice. Neurosci Lett 459:66–68
Araujo GG, Papoti M, Reis IG, Mello MA, Gobatto CA (2012) Physiological responses during linear periodized training in rats. Eur J Appl Physiol;112:839-52
El-Sayes J, Harasym D, Turco CV, Locke MB, Nelson AJ (2019) Exercise-induced neuroplasticity: a mechanistic model and prospects for promoting plasticity. Neuroscientist. ;25(1):65-85
Cechella JL, Leite MR, Gai RM, Zeni G (2014) The impact of a diphenyl diselenide-supplemented diet and aerobic exercise on memory of middle-aged rats. Physiol Behav;135:125–9
Lawler JM (2011) Exacerbation of pathology by oxidative stress in respiratory and locomotor muscles with Duchenne muscular dystrophy. J Physiol 589:2161–2170
Moraes LH, Bollineli RC, Mizobuti DS, dos Silveira L, R, Marques MJ, Minatel E. (2015) Effect of N-acetylcysteine plus deferoxamine on oxidative stress and inflammation in dystrophic muscle cells. Redox Rep 2015(20):109–115
Kozakowska M, Pietraszek-Gremplewicz K, Jozkowicz A, Dulak J (2015) The role of oxidative stress in skeletal muscle injury and regeneration: focus on antioxidant enzymes. J Muscle Res Cell Motil 36:377–393
Gomez-Cabrera MC, Domenech E, Viña J (2008) Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med 44:126–131
Antoncic-Svetina M, Sentija D, Cipak A, Milicic D, Meinitzer A, Tatzber F (2010) Ergometry induces systemic oxidative stress in healthy human subjects. Tohoku J Exp Med 221:43–48
Jia B, Wang X, Kang A, Wang X, Wen Z, Yao W et al (2012) The effects of long term aerobic exercise. Clin Hemorheol Microcirc 51:117–127
Halliwell B (1992) (1992) Reactive oxygen species and the central nervous system. J Neurochem 59:609–623
Funding
This research was supported by grants from Unisul (CMC) and the UNIEDU/FUMDES (AA).
Author information
Authors and Affiliations
Contributions
Priscila Mantovani Nocetti, Viviane Freiberger, and Letícia Ventura planned the experiments and wrote the paper. Clarissa Martinelli Comim managed the project and performed most of the experiments and analysis and provided input on statistical analysis and interpretation. Adriano Alberti provided substantial input for the manuscript and wrote the paper. Daniel Fernandes Martins, Leoberto Ricardo Grigollo, Cristina Salar Andreau, and Rudy José Nodari Junior provided technical guidance and supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nocetti, P.M., Alberti, A., Freiberger, V. et al. Swimming Improves Memory and Antioxidant Defense in an Animal Model of Duchenne Muscular Dystrophy. Mol Neurobiol 58, 5067–5077 (2021). https://doi.org/10.1007/s12035-021-02482-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02482-y