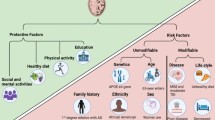
Abstract
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by an increasing deterioration of memory, which is concomitant with additional cognitive deficits. Neurofibrillary tangles and senile plaques are two pivotal proteins inside the brain that are considered essential to obstruct the normal cognitive function of the brain. Genetic variations in TREM2 gene disturb the neuroinflammatory action of microglia in reducing the progression of the disease.
TREM2 is a transmembrane receptor present on the microglia, which has an important function in neuroinflammation. Genome-wide association studies identified variants of TREM2 gene and linked it with the risk of developing AD, by 2–4 folds. Numerous studies on mice models have revealed the relationship between mutations of TREM2 gene and its effect on amyloid burden and tau pathology in the brain that gets affected by AD. This review summarizes the role of TREM2 and its variants in the progression of AD and tries to delve deep into the role of soluble TREM2 as an effective biomarker and impending neuroprotection in AD. It also focuses on the strategies to develop therapeutic agents against TREM2 by employing its expression, function, and signalling pathways. The current challenges posed against prospective therapy for AD are also discussed.
Graphical abstract




Similar content being viewed by others
Data Availability
All data and material are now available.
References
Zhong L, Xu Y, Zhuo R, Wang T, Wang K, Huang R, Wang D, Gao Y, Zhu Y, Sheng X, Chen K, Wang N, Zhu L, Can D, Marten Y, Shinohara M, Liu CC, Du D, Sun H, Wen L, Xu H, Bu G, Chen XF (2019) Soluble TREM2 ameliorates pathological phenotypes by modulating microglial functions in an Alzheimer’s disease model. Nat Commun 10:1–16. https://doi.org/10.1038/s41467-019-09118-9
Ulrich JD, Ulland TK, Colonna M, Holtzman DM (2017) Elucidating the role of TREM2 in Alzheimer’s disease. Neuron 94:237–248. https://doi.org/10.1016/j.neuron.2017.02.042
Campion D, Dumanchin C, Hannequin D, Dubois B, Belliard S, Puel M, Thomas-Anterion C, Michon A, Martin C, Charbonnier F, Raux G, Camuzat A, Penet C, Mesnage V, Martinez M, Clerget-Darpoux F, Brice A, Frebourg T (1999) Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. Am J Hum Genet https://doi.org/10.1086/302553
Guerreiro RJ, Gustafson DR, Hardy J (2012) The genetic architecture of Alzheimer’s disease: Beyond APP, PSENS and APOE. Neurobiol Aging https://doi.org/10.1016/j.neurobiolaging.2010.03.025
Wolfe CM, Fitz NF, Nam KN, Lefterov I, Koldamova R (2019) The role of APOE and TREM2 in Alzheimer ′ s disease—Current understanding and perspectives. Int J Mol Sci 20:65–70. https://doi.org/10.3390/ijms20010081
Wakselman S, Béchade C, Roumier A, Bernard D, Triller A, Bessis A (2008) Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci. https://doi.org/10.1523/JNEUROSCI.1006-08.2008
Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci https://doi.org/10.1038/nn1472
Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I (2017) A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. https://doi.org/10.1016/j.cell.2017.05.018
Rangaraju S, Dammer EB, Raza SA, Rathakrishnan P, Xiao H, Gao T, Duong DM, Pennington MW, Lah JJ, Seyfried NT, Levey AI (2018) Identification and therapeutic modulation of a pro-inflammatory subset of disease-associated-microglia in Alzheimer’s disease. Mol Neurodegener. https://doi.org/10.1186/s13024-018-0254-8
Gratuze M, Leyns CEG, Holtzman DM (2018) New insights into the role of TREM2 in Alzheimer’s disease. Mol Neurodegener 13:1–16. https://doi.org/10.1186/s13024-018-0298-9
Carmona S, Zahs K, Wu E, Dakin K, Bras J, Guerreiro R (2018) The role of TREM2 in Alzheimer’s disease and other neurodegenerative disorders. Lancet Neurol 17:721–730. https://doi.org/10.1016/S1474-4422(18)30232-1
Ulrich JD, Holtzman DM (2016) TREM2 function in Alzheimer’s disease and neurodegeneration. ACS Chem Neurosci 7:420–427. https://doi.org/10.1021/acschemneuro.5b00313
Heneka MT, Carson MJ, Khoury JE, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14:388–405. https://doi.org/10.1016/S1474-4422(15)70016-5
Zhu M, Wang X, Sun L, Schultzberg M, Hjorth E (2018) Can inflammation be resolved in Alzheimer’s disease? Ther Adv Neurol Disord. https://doi.org/10.1177/1756286418791107
Gussago C, Casati M, Ferri E, Arosio B (2019) The triggering receptor expressed on myeloid cells-2 (TREM-2) as expression of the relationship between microglia and Alzheimer’s disease: a novel marker for a promising pathway to explore. J Frailty Aging 8 54–56. https://doi.org/10.14283/jfa.2018.43
Sarlus H, Heneka MT (2017) Microglia in Alzheimer’s disease. J Clin Invest. https://doi.org/10.1172/JCI90606
Streit WJ, Xue Q-S, Tischer J., Bechmann I (2014) Microglial pathology. Acta Neuropathol Commun. https://doi.org/10.1186/preaccept-1035265697142235
Perry VH, Holmes C (2014) Microglial priming in neurodegenerative disease. Nat Rev Neurol https://doi.org/10.1038/nrneurol.2014.38
Hampel H, Caraci F, Cuello AC, Caruso G, Nisticò R, Corbo M, Baldacci F, Toschi N, Garaci F, Chiesa PA, Verdooner SR, Akman-Anderson L, Hernández F, Ávila J, Emanuele E, Valenzuela PL, Lucía, A., Watling, M., Imbimbo, B.P., Vergallo, A., Lista, S., 2020. A path toward precision medicine for neuroinflammatory mechanisms in Alzheimer’s disease. Front Immunol. https://doi.org/10.3389/fimmu.2020.00456
Tang ZH, Li T, Tong YG, Chen XJ, Chen XP, Wang YT, Lu JJ (2015) A Systematic Review of the Anticancer Properties of Compounds Isolated from Licorice (Gancao). Planta Med. https://doi.org/10.1055/s-0035-1558227
Edwards FA (2019) A unifying hypothesis for Alzheimer’s disease: from plaques to neurodegeneration. Trends Neurosci. https://doi.org/10.1016/j.tins.2019.03.003
Perea JR, Ávila J, Bolós M (2018) Dephosphorylated rather than hyperphosphorylated Tau triggers a pro-inflammatory profile in microglia through the p38 MAPK pathway. Exp Neurol https://doi.org/10.1016/j.expneurol.2018.08.007
Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, Bianchin M, Bird T, Miranda R, Salmaggi A, Tranebjærg L, Konttinen Y, Peltonen L (2002) Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet https://doi.org/10.1086/342259
Colonna M, Wang Y (2016) TREM2 variants: new keys to decipher Alzheimer disease pathogenesis. Nat Rev Neurosci https://doi.org/10.1038/nrn.2016.7
Otero K, Turnbull IR, Poliani PL, Vermi W, Cerutti E, Aoshi T, Tassi I, Takai T, Stanley SL, Miller M, Shaw AS, Colonna M (2009) Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and β-catenin. Nat Immunol. https://doi.org/10.1038/ni.1744
Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, Holtzman DM, Cirrito JR, Colonna M (2015) TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell. https://doi.org/10.1016/j.cell.2015.01.049
Takahashi K, Rochford CDP, Neumann H (2005) Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. https://doi.org/10.1084/jem.20041611
Ito H, Hamerman JA (2012) TREM-2, triggering receptor expressed on myeloid cell-2, negatively regulates TLR responses in dendritic cells. Eur J Immunol 42:176–185. https://doi.org/10.1002/eji.201141679
Yeh FL, Hansen DV, Sheng M (2017) TREM2, microglia, and neurodegenerative diseases. Trends Mol Med. https://doi.org/10.1016/j.molmed.2017.03.008
Ulrich JD, Ulland TK, Mahan TE, Nyström S, Peter Nilsson K, Song WM, Zhou Y, Reinartz M, Choi S, Jiang H, Stewart FR, Anderson E, Wang Y, Colonna M, Holtzman DM (2018) ApoE facilitates the microglial response to amyloid plaque pathology. J Exp Med. https://doi.org/10.1084/jem.20171265
Kober DL, Stuchell-Brereton MD, Kluender CE, Dean HB, Strickland MR, Steinberg DF, Nelson SS, Baban B, Holtzman DM, Frieden C, Alexander-Brett J, Roberson ED, Song Y, Brett TJ (2020) Functional insights from biophysical study of TREM2 interactions with apoE and Aβ1-42. Alzheimer’s Dement. https://doi.org/10.1002/alz.12194
Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JSK, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St. George-Hyslop P, Singleton A, Hardy J (2013a) TREM2 Variants in Alzheimer’s Disease . N Engl J Med 368, 117–127. https://doi.org/10.1056/nejmoa1211851
Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K (2013) Variant of TREM2 Associated with the Risk of Alzheimer’s Disease. N Engl J Med 368:107–116. https://doi.org/10.1056/nejmoa1211103
Forabosco P, Ramasamy A, Trabzuni D, Walker R, Smith C, Bras J, Levine AP, Hardy J, Pocock JM, Guerreiro R, Weale ME, Ryten M (2013) Insights into TREM2 biology by network analysis of human brain gene expression data. Neurobiol Aging https://doi.org/10.1016/j.neurobiolaging.2013.05.001
Zheng H, Cheng B, Li Y, Li X, Chen X, Zhang Y (2018) TREM2 in Alzheimer’s disease: microglial survival and energy metabolism. Front Aging Neurosci 10:1–10. https://doi.org/10.3389/fnagi.2018.00395
Lee CYD, Daggett A, Gu X, Jiang LL, Langfelder P, Li X, Wang N, Zhao Y, Park CS, Cooper Y, Ferando I, Mody I, Coppola G, Xu H, Yang XW (2018) Elevated TREM2 gene dosage reprograms microglia responsivity and ameliorates pathological phenotypes in Alzheimer’s disease models. Neuron. https://doi.org/10.1016/j.neuron.2018.02.002
Wang Y, Ulland TK, Ulrich JD, Song W, Tzaferis JA, Hole JT, Yuan P, Mahan TE, Shi Y, Gilfillan S, Cella M, Grutzendler J, DeMattos RB, Cirrito JR, Holtzman DM, Colonna M (2016) TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J Exp Med 213:667–675. https://doi.org/10.1084/jem.20151948
Bemiller SM, McCray TJ, Allan K, Formica SV, Xu G, Wilson G, Kokiko-Cochran ON, Crish SD, Lasagna-Reeves CA, Ransohoff RM, Landreth GE, Lamb BT (2017) TREM2 deficiency exacerbates tau pathology through dysregulated kinase signaling in a mouse model of tauopathy. Mol Neurodegener https://doi.org/10.1186/s13024-017-0216-6
Leyns CEG, Holtzman DM (2017) Glial contributions to neurodegeneration in tauopathies. Mol Neurodegener https://doi.org/10.1186/s13024-017-0192-x
Sayed FA, Telpoukhovskaia M, Kodama L, Li Y, Zhou Y, Le D, Hauduc A, Ludwig C, Gao F, Clelland C, Zhan L, Cooper YA, Davalos D, Akassoglou K, Coppola G, Gan L (2018) Differential effects of partial and complete loss of TREM2 on microglial injury response and tauopathy. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.1811411115
Jay TR, Hirsch AM, Broihier ML, Miller CM, Neilson LE, Ransohoff RM, Lamb BT, Landreth GE (2017) Disease progression-dependent effects of TREM2 deficiency in a mouse model of Alzheimer’s disease. J Neurosci. https://doi.org/10.1523/JNEUROSCI.2110-16.2016
Zheng H, Liu CC, Atagi Y, Chen XF, Jia L, Yang L, He W, Zhang X, Kang SS, Rosenberry TL, Fryer JD, Zhang YW, Xu H, Bu G (2016) Opposing roles of the triggering receptor expressed on myeloid cells 2 and triggering receptor expressed on myeloid cells-like transcript 2 in microglia activation. Neurobiol Aging. https://doi.org/10.1016/j.neurobiolaging.2016.03.004
Zhong L, Chen XF, Zhang ZL, Wang Z, Shi XZ, Xu K, Zhang YW, Xu H, Bu G (2015) DAP12 stabilizes the C-terminal fragment of the triggering receptor expressed on myeloid cells-2 (TREM2) and protects against LPS-induced pro-inflammatory response. J Biol Chem. https://doi.org/10.1074/jbc.M115.645986
Zhong L, Zhang ZL, Li X, Liao C, Mou P, Wang T, Wang Z, Wang Z, Wei M, Xu H, Bu G, Chen XF (2017) TREM2/DAP12 complex regulates inflammatory responses in Microglia via the JNK signaling pathway. Front. Aging Neurosci. https://doi.org/10.3389/fnagi.2017.00204
Zhao Y, Li X, Huang T, Jiang LL, Tan Z, Zhang M, Cheng IHJ Wang X, Bu G, Zhang YW, Wang Q, Xu H (2017) Intracellular trafficking of TREM2 is regulated by presenilin 1. Exp Mol Med. https://doi.org/10.1038/emm.2017.200
Matarin M, Salih DA, Yasvoina M, Cummings DM, Guelfi S, Liu W, NahabooSolim MA, Moens TG, Paublete RM, Ali SS, Perona M, Desai R, Smith KJ, Latcham J, Fulleylove M, Richardson JC, Hardy J, Edwards FA (2015) A Genome-wide gene-expression analysis and database in transgenic mice during development of amyloid or tau pathology. Cell Rep. https://doi.org/10.1016/j.celrep.2014.12.041
Soragna D (2003) An Italian family affected by Nasu-Hakola disease with a novel genetic mutation in the TREM2 gene. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp.74.6.825-a
Kober DL, Alexander-Brett JM, Karch CM, Cruchaga C, Colonna M, Holtzman MJ, Brett TJ (2016) Neurodegenerative disease mutations in TREM2 reveal a functional surface and distinct loss-of-function mechanisms. Elife. https://doi.org/10.7554/eLife.20391
Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JSK, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St. George-Hyslop P, Singleton A, Hardy J (2013b) TREM2 variants in Alzheimer’s Disease . N Engl J Med https://doi.org/10.1056/nejmoa1211851
Jin SC, Benitez BA, Karch CM, Cooper B, Skorupa T, Carrell D, Norton JB, Hsu S, Harari O, Cai Y, Bertelsen S, Goate AM, Cruchaga C (2014) Coding variants in TREM2 increase risk for Alzheimer’s disease. Hum Mol Genet. https://doi.org/10.1093/hmg/ddu277
Kleinberger G, Yamanishi Y, Suárez-Calvet M, Czirr E, Lohmann E, Cuyvers E, Struyfs H, Pettkus N, Wenninger-Weinzierl A, Mazaheri F, Tahirovic S, Lleó A, Alcolea D, Fortea J, Willem M, Lammich S, Molinuevo JL, Sánchez-Valle R, Antonell A, Ramirez A, Heneka MT, Sleegers K, Van Der Zee J, Martin JJ, Engelborghs S, Demirtas-Tatlidede A, Zetterberg H, Van Broeckhoven C, Gurvit H, Wyss-Coray T, Hardy J, Colonna M, Haass C (2014) TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci Transl Med https://doi.org/10.1126/scitranslmed.3009093
Ulrich JD, Finn MB, Wang Y, Shen A, Mahan TE, Jiang H, Stewart, FR, Piccio L, Colonna M, Holtzman DM (2014) Altered microglial response to Aβ plaques in APPPS1–21 mice heterozygous for TREM2. Mol. Neurodegener. https://doi.org/10.1186/1750-1326-9-20
Jay TR, Von Saucken VE, Landreth GE (2017b) TREM2 in Neurodegenerative Diseases. Mol Neurodegener. https://doi.org/10.1186/s13024-017-0197-5
Song W, Hooli B, Mullin K, Jin SC, Cella M, Ulland TK, Wang Y, Tanzi RE, Colonna M (2017) Alzheimer’s disease-associated TREM2 variants exhibit either decreased or increased ligand-dependent activation. Alzheimer’s Dement. https://doi.org/10.1016/j.jalz.2016.07.004
Sudom A, Talreja S, Danao J, Bragg E, Kegel R, Min X, Richardson J, Zhang Z, Sharkov N, Marcora E, Thibault S, Bradley J, Wood S, Lim A-C, Chen H, Wang S, Foltz IN, Sambashivan S, Wang Z (2018) Molecular basis for the loss-of-function effects of the Alzheimer’s disease–associated R47H variant of the immune receptor TREM2. J Biol Chem 293:12634–12646. https://doi.org/10.1074/jbc.ra118.002352
Radde R, Bolmont T, Kaeser SA, Coomaraswamy J, Lindau D, Stoltze L, Calhoun ME, Jäggi F, Wolburg H, Gengler S, Haass C, Ghetti B, Czech C, Hölscher C, Mathews PM, Jucker M (2006) Aβ42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. https://doi.org/10.1038/sj.embor.7400784
Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R (2006) Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J Neurosci. https://doi.org/10.1523/JNEUROSCI.1202-06.2006
Ma L, Allen M, Sakae N, Ertekin-Taner N, Graff-Radford NR, Dickson DW, Younkin SG, Sevlever D (2016) Expression and processing analyses of wild type and p.R47H TREM2 variant in Alzheimer’s disease brains. Mol Neurodegener https://doi.org/10.1186/s13024-016-0137-9
Piccio L, Deming Y, Del-Águila JL, Ghezzi L, Holtzman DM, Fagan AM, Fenoglio C, Galimberti D, Borroni B, Cruchaga C (2016) Cerebrospinal fluid soluble TREM2 is higher in Alzheimer disease and associated with mutation status. Acta Neuropathol. https://doi.org/10.1007/s00401-016-1533-5
Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, Wolozin B, Butovsky O, Kügler S, Ikezu T (2015) Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci https://doi.org/10.1038/nn.4132
Luo W, Liu W, Hu X, Hanna M, Caravaca A, Paul SM (2015) Microglial internalization and degradation of pathological tau is enhanced by an anti-tau monoclonal antibody. Sci Rep https://doi.org/10.1038/srep11161
Piccio L, Buonsanti C, Cella M, Tassi I, Schmidt RE, Fenoglio C, Rinker J, Naismith RT, Panina-Bordignon P, Passini N, Galimberti D, Scarpini E, Colonna M, Cross AH (2008) Identification of soluble TREM-2 in the cerebrospinal fluid and its association with multiple sclerosis and CNS inflammation. Brain. https://doi.org/10.1093/brain/awn217
Heslegrave A, Heywood W, Paterson R, Magdalinou N, Svensson J, Johansson P, Öhrfelt A, Blennow K, Hardy J, Schott J, Mills K, Zetterberg H (2016) Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer’s disease. Mol Neurodegener https://doi.org/10.1186/s13024-016-0071-x
Suárez‐Calvet M, Kleinberger G, Araque Caballero MÁ, Brendel M, Rominger A, Alcolea D, Fortea J, Lleó A, Blesa R, Gispert JD, Sánchez‐Valle R, Antonell A, Rami L, Molinuevo JL, Brosseron F, Traschütz A, Heneka MT, Struyfs H, Engelborghs S, Sleegers K, Van Broeckhoven C, Zetterberg H, Nellgård B, Blennow K, Crispin A, Ewers M, Haass C (2016) TREM 2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early‐stage Alzheimer’s disease and associate with neuronal injury markers . EMBO Mol Med https://doi.org/10.15252/emmm.201506123
Bergles DE, Richardson WD (2016) Oligodendrocyte development and plasticity. Cold Spring Harb Perspect Biol https://doi.org/10.1101/cshperspect.a020453
Biber K, Bhattacharya A, Campbell BM, Piro JR, Rohe M, Staal RGW, Talanian RV, Möller T (2019) Microglial drug targets in AD: Opportunities and challenges in drug discovery and development. Front Pharmacol https://doi.org/10.3389/fphar.2019.00840
Biber K, Möller T, Boddeke E, Prinz M (2016) Central nervous system myeloid cells as drug targets: Current status and translational challenges. Nat Rev Drug Discov. https://doi.org/10.1038/nrd.2015.14
Heppner FL, Ransohoff RM, Becher B (2015) Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci https://doi.org/10.1038/nrn3880
Osborn LM, Kamphuis W, Wadman WJ, Hol EM (2016) Astrogliosis: an integral player in the pathogenesis of Alzheimer’s disease. Prog Neurobiol. https://doi.org/10.1016/j.pneurobio.2016.01.001
Raj DDA, Jaarsma D, Holtman IR, Olah M, Ferreira FM, Schaafsma W, Brouwer N, Meijer MM, De Waard MC, Van der Pluijm I, Brandt R, Kreft KL, Laman JD, De Haan G, Biber KPH, Hoeijmakers JHJ, Eggen BJL, Boddeke HWGM (2014) Priming of microglia in a DNA-repair deficient model of accelerated aging. Neurobiol Aging https://doi.org/10.1016/j.neurobiolaging.2014.03.025
Ransohoff RM, El Khoury J (2016) Microglia in health and disease. Cold Spring Harb Perspect Biol https://doi.org/10.1101/cshperspect.a020560
Zuchero JB, Barres BA (2015) Glia in mammalian development and disease. Dev. https://doi.org/10.1242/dev.129304
Atagi Y, Liu CC, Painter MM, Chen XF, Verbeeck C, Zheng H, Li X, Rademakers R, Kang SS, Xu H, Younkin S, Das P, Fryer JD, Bu G (2015) Apolipoprotein E is a ligand for triggering receptor expressed on myeloid cells 2 (TREM2). J Biol Chem. https://doi.org/10.1074/jbc.M115.679043
Lessard CB, Malnik SL, Zhou Y, Ladd TB, Cruz PE, Ran Y, Mahan TE, Chakrabaty P, Holtzman DM, Ulrich JD, Colonna M, Golde TE, 2018. High‐affinity interactions and signal transduction between Aβ oligomers and TREM 2. EMBO Mol Med https://doi.org/10.15252/emmm.201809027
Yeh FL, Wang Y, Tom I, Gonzalez LC, Sheng M (2016) TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron. https://doi.org/10.1016/j.neuron.2016.06.015
Zhong L, Wang Z, Wang D, Wang Z, Martens YA, Wu L, Xu, Y. Wang K, Li J, Huang R, Can D, Xu H, Bu G, Chen, XF (2018) Amyloid-beta modulates microglial responses by binding to the triggering receptor expressed on myeloid cells 2 (TREM2). Mol Neurodegener https://doi.org/10.1186/s13024-018-0247-7
Kober DL, Brett TJ (2017) TREM2-Ligand Interactions in Health and Disease. J Mol Biol. https://doi.org/10.1016/j.jmb.2017.04.004
Shi Y, Holtzman DM (2018) Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat Rev Immunol. https://doi.org/10.1038/s41577-018-0051-1
Bohlen CJ, Bennett FC, Tucker AF, Collins HY, Mulinyawe SB, Barres BA (2017) Diverse requirements for microglial survival, specification, and function revealed by defined-medium cultures. Neuron. https://doi.org/10.1016/j.neuron.2017.04.043
Deczkowska A, Weiner A, Amit I (2020) The physiology, pathology, and potential therapeutic applications of the TREM2 signaling pathway. Cell. https://doi.org/10.1016/j.cell.2020.05.003
Schlepckow K, Kleinberger G, Fukumori A, Feederle R, Lichtenthaler SF, Steiner H, Haass C (2017) An Alzheimer‐associated TREM2 variant occurs at the ADAM cleavage site and affects shedding and phagocytic function . EMBO Mol Med. https://doi.org/10.15252/emmm.201707672
Schlepckow K, Monroe KM, Kleinberger G, Cantuti‐Castelvetri L, Parhizkar S, Xia D, Willem M, Werner G, Pettkus N, Brunner B, Sülzen A, Nuscher B, Hampel H, Xiang X, Feederle R, Tahirovic S, Park JI, Prorok R, Mahon C, Liang C, Shi J, Kim DJ, Sabelström H, Huang F, Di Paolo G, Simons M, Lewcock JW, Haass C (2020) Enhancing protective microglial activities with a dual function TREM 2 antibody to the stalk region. EMBO Mol Med. https://doi.org/10.15252/emmm.201911227
Author information
Authors and Affiliations
Contributions
All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
All authors agreed with the submitted version of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kulkarni, B., Kumar, D., Cruz-Martins, N. et al. Role of TREM2 in Alzheimer’s Disease: A Long Road Ahead. Mol Neurobiol 58, 5239–5252 (2021). https://doi.org/10.1007/s12035-021-02477-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02477-9