Abstract
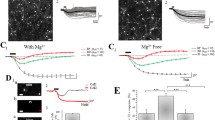
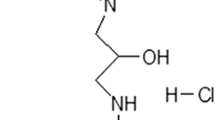
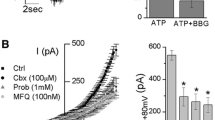
Dexpramipexole (DEX) has been described as the first-in-class F1Fo ATP synthase activator able to boost mitochondrial bioenergetics and provide neuroprotection in experimental models of ischemic brain injury. Although DEX failed in a phase III trial in patients with amyotrophic lateral sclerosis, it showed favorable safety and tolerability profiles. Recently, DEX emerged as a Nav1.8 Na+ channel and transient outward K+ (IA) conductance blocker, revealing therefore an unexpected, pleiotypic pharmacodynamic profile. In this study, we performed electrophysiological experiments in vitro aimed to better characterize the impact of DEX on voltage-dependent currents and synaptic transmission in the hippocampus. By means of patch-clamp recordings on isolated hippocampal neurons, we found that DEX increases outward K+ currents evoked by a voltage ramp protocol. This effect is prevented by the non-selective voltage-dependent K+ channel (Kv) blocker TEA and by the selective small-conductance Ca2+-activated K+ (SK) channel blocker apamin. In keeping with this, extracellular field recordings from rat hippocampal slices also demonstrated that the compound inhibits synaptic transmission and CA1 neuron excitability. Overall, these data further our understanding on the pharmacodynamics of DEX and disclose an additional mechanism that could underlie its neuroprotective properties. Also, they identify DEX as a lead to develop new modulators of K+ conductances.





Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Alavian KN, Dworetzky SI, Bonanni L, Zhang P, Sacchetti S, Li HM, Signore AP, Smith PJS et al (2015) The mitochondrial complex V-associated large-conductance inner membrane current is regulated by cyclosporine and dexpramipexole. Mol Pharmacol 87:1–8
Alavian KN, Dworetzky SI, Bonanni L, Zhang P, Sacchetti S, Mariggio MA, Onofrj M, Thomas A et al (2012) Effects of dexpramipexole on brain mitochondrial conductances and cellular bioenergetic efficiency. Brain Res 1446:1–11
Anderson WW, Collingridge GL (2001) The LTP Program: a data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J Neurosci Methods 108:71–83
Baj G, Patrizio A, Montalbano A, Sciancalepore M, Tongiorgi E (2014) Developmental and maintenance defects in Rett syndrome neurons identified by a new mouse staging system in vitro. Front Cell Neurosci:8
Barry PH (1994) JPCALC, a software package for calculating liquid-junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods 51:107–116
Cassarino DS, Fall CP, Smith TS, Bennett JP (1998) Pramipexole reduces reactive oxygen species production in vivo and in vitro and inhibits the mitochondrial permeability transition produced by the Parkinsonian neurotoxin methylpyridinium ion. J Neurochem 71:295–301
Cheah BC, Kiernan MC (2010) Dexpramipexole, the R(+) enantiomer of pramipexole, for the potential treatment of amyotrophic lateral sclerosis. Idrugs 13:911–920
Chiarugi A (2002) Characterization of the molecular events following impairment of NF-kappa B-driven transcription in neurons. Mol Brain Res 109:179–188
Cifra A, Mazzone GL, Nistri A (2013) Riluzole: what it does to spinal and brainstem neurons and how it does it. Neuroscientist 19:137–144
Colotta V, Lenzi O, Catarzi D, Varano F, Squarcialupi L, Costagli C, Galli A, Ghelardini C et al (2012) 3-Hydroxy-1H-quinazoline-2,4-dione derivatives as new antagonists at ionotropic glutamate receptors: molecular modeling and pharmacological studies. Eur J Med Chem 54:470–482
Coppi E, Cellai L, Maraula G, Dettori I, Melani A, Pugliese AM, Pedata F (2015) Role of adenosine in oligodendrocyte precursor maturation. Front Cell Neurosci 9
Coppi E, Cherchi F, Fusco I, Failli P, Vona A, Dettori I, Gaviano L, Lucarini E et al (2019) Adenosine A(3) receptor activation inhibits pronociceptive N-type Ca2+ currents and cell excitability in dorsal root ganglion neurons. Pain 160:1103–1118
Coppi E, Lana D, Cherchi F, Fusco I, Buonvicino D, Urru M, Ranieri G, Muzzi M et al (2018) Dexpramipexole enhances hippocampal synaptic plasticity and memory in the rat. Neuropharmacology 143:306–316
Coppi E, Pedata F, Gibb AJ (2012) P2Y(1) receptor modulation of Ca2 + -activated K+ currents in medium-sized neurons from neonatal rat striatal slices. J Neurophysiol 107:1009–1021
Cudkowicz M, Bozik ME, Ingersoll EW, Miller R, Mitsumoto H, Shefner J, Moore DH, Schoenfeld D et al (2011) The effects of dexpramipexole (KNS-760704) in individuals with amyotrophic lateral sclerosis. Nat Med 17:1652–U1169
Cudkowicz M, Van den Berg L, Shefner J, Mitsumoto H, Mora J, Ludolph A, Hardiman O, Ingersoll E et al (2013) Efficacy of Dexpramipexole in amyotrophic lateral sclerosis: data from the phase III EMPOWER trial. Neurology 80
Cudkowicz ME, van den Berg LH, Shefner JM, Mitsumoto H, Mora JS, Ludolph A, Hardiman O, Bozik ME et al (2013) Dexpramipexole versus placebo for patients with amyotrophic lateral sclerosis (EMPOWER): a randomised, double-blind, phase 3 trial. Lancet Neurol 12:1059–1067
Danzeisen R, Schwalenstoecker B, Gillardon F, Buerger E, Krzykalla V, Klinder K, Schild L, Hengerer B et al (2006) Targeted antioxidative and neuroprotective properties of the dopamine agonist pramipexole and its nondopaminergic enantiomer SND919CL2x (+)2-amino-4,5,6,7-tetrahydro-6-L-propylamino-benzathiazole dihydrochloride. J Pharmacol Exp Ther 316:189–199
Dworetzky SI, Hebrank GT, Archibald DG, Reynolds IJ, Farwell W, Bozik ME (2017) The targeted eosinophil-lowering effects of dexpramipexole in clinical studies. Blood Cells Mol Dis 63:62–65
Frey U, Schroeder H, Matthies H (1990) Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Res 522:69–75
Fusco I, Ugolini F, Lana D, Coppi E, Dettori I, Gaviano L, Nosi D, Cherchi F et al (2018) The selective antagonism of adenosine A(2B) receptors reduces the synaptic failure and neuronal death induced by oxygen and glucose deprivation in rat CA1 hippocampus in vitro. Front Pharmacol 9
Jonas EA, Porter GA, Beutner G, Mnatsakanyan N, Alavian KN (2015) Cell death disguised: the mitochondrial permeability transition pore as the c-subunit of the F1FO ATP synthase. Pharmacol Res 99:382–392
Lee SI, Hoeijmakers JGJ, Faber CG, Merkies ISJ, Lauria G, Waxman SG (2020) The small fiber neuropathy NaV1.7 I228M mutation: impaired neurite integrity via bioenergetic and mitotoxic mechanisms, and protection by dexpramipexole. J Neurophysiol 123:645–657
Li-Smerin Y, Swartz KJ (1998) Gating modifier toxins reveal a conserved structural motif in voltage-gated Ca2+ and K+ channels. Proc Natl Acad Sci U S A 95:8585–8589
Licznerski P, Park HA, Rolyan H, Chen RM, Mnatsakanyan N, Miranda P, Graham M, Wu J et al (2020) ATP synthase c-subunit leak causes aberrant cellular metabolism in fragile X syndrome. Cell 182:1170-+
Muzzi M, Buonvicino D, Urru M, Tofani L, Chiarugi A (2018) Repurposing of dexpramipexole to treatment of neonatal hypoxic/ischemic encephalopathy. Neurosci Lett 687:234–240
Muzzi M, Gerace E, Buonvicino D, Coppi E, Resta F, Formentini L, Zecchi R, Tigli L et al (2018) Dexpramipexole improves bioenergetics and outcome in experimental stroke. Br J Pharmacol 175:272–283
Samano C, Nistri A (2019) Mechanism of neuroprotection against experimental spinal cord injury by riluzole or methylprednisolone. Neurochem Res 44:200–213
Sayeed I, Parvez S, Winkler-Stuck K, Seitz G, Trieu I, Wallesch CW, Schonfeld P, Siemen D (2006) Patch clamp reveals powerful blockade of the mitochondrial permeability transition pore by the D2-receptor agonist pramipexole. FASEB J 20:556-+
Urru M, Muzzi M, Coppi E, Ranieri G, Buonvicino D, Camaioni E, Coppini R, Pugliese AM et al (2020) Dexpramipexole blocks Na(v)1.8 sodium channels and provides analgesia in multiple nociceptive and neuropathic pain models. Pain 161:831–841
Funding
The present work was supported by the University of Florence (Fondi Ateneo), by grants from Italian Foundation for Multiple Sclerosis (FISM) (2019/R-Single/036 (AMP and EC) and 2014/R/6 (AC)); by Fondazione Umberto Veronesi (EC), by PRIN 2017 (AC), Regione Toscana Progetto Salute 2018 (AC), AIRC, and Fondazione CR Firenze under IG 2017 - ID. 2045 (AC).
Author information
Authors and Affiliations
Contributions
EC and AC made substantial contributions to the conception or design of the work; EC, DB, GR, FC, and MV performed the experiments and analyzed data; EC, AMP, and AC worked on interpretation of data. EC drafted the work; AC and AMP revised it critically; AC approved the version to be published.
Corresponding author
Ethics declarations
The present research does not involve human participants. All animal procedures were conducted according to the Italian Guidelines for Animal Care, DL 26/2014, and authorized by the Italian Ministry of Health, Aut. N. 788/2016-PR.
Conflict of Interest
The authors declare that they have no conflict of interest.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Coppi, E., Buonvicino, D., Ranieri, G. et al. Dexpramipexole Enhances K+ Currents and Inhibits Cell Excitability in the Rat Hippocampus In Vitro. Mol Neurobiol 58, 2955–2962 (2021). https://doi.org/10.1007/s12035-021-02300-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02300-5