Abstract
Amyloid-β (Aβ), the influence of which is considered the pathomechanism of Alzheimer’s disease, is also present in healthy people. The microbiome’s impact is also taken into account, where bacterial lipopolysaccharide (LPS) activates inflammatory processes and stimulates microglia via TLRs. Molecules of bacterial origin can co-create senile plaques with Aβ. This study evaluated the activity of physiological Aβ concentrations on neuronal and microglial cells after preincubation with LPS. Two cell lines were used in the study: PC12 cells differentiated with NGF and THP-1 cells differentiated with phorbol 12-myristate 13-acetate (PMA). Cells were incubated with LPS at concentrations of 1–100 μM for 24 h and then with Aβ25–35 at a concentration of 0.001 μM or 1.0 μM for another 24 h. The viability of the culture and free oxygen radicals and the number of DNA strand breaks in both cell lines were evaluated. Additionally, for PC12 cells, neural features were assessed. Stimulation of repair processes in the presence of Aβ was observed for both studied cell lines. There was a decrease in free radical level and DNA damage number compared to control cultures (cells treated with LPS and without Aβ). The neurotrophic activity of Aβ was observed—the effect on neurites’ growth even after the preincubation of PC12 cells with LPS. At the lowest concentration of LPS used, the increase in neurite length was about 50% greater than in the negative control. At low concentrations, Aβ has a protective effect on neuron-like PC12 cells pretreated with LPS.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is classified as a neurodegenerative disorder. This disease includes pathological loss of nerve cells and a reduction in the number of synaptic connections along with the loss of their plasticity. The disease develops asymptomatically for many years, and as a consequence, changes in the brain cause difficulties in daily activities, memory loss, mood swings, and changes in behavior. Over time, problems with recalling words or numbers and even disorientation in the family environment appear. The progress of science and technology influenced the development of medicine, the life span of people has significantly increased, and the risk of developing AD increases with age. Due to the growing number of patients, the financial outlay for medical care is growing and searching for possible effective therapies. There are still no effective drugs—the currently used drugs can only slow down the disease’s progression and do not reverse the changes that have already taken place. It is also a socially burdensome problem, and for family members.
The pathomechanism of AD is also ambiguous. In the literature, we can read many hypotheses about the development of the disease. The oldest of them is the cholinergic hypothesis. Currently used drugs contain active substances that inhibit the activity of acetylcholinesterase. The next two most popular are the tau protein hyperphosphorylation and the amyloid hypothesis—based on the toxicity of amyloid-β (Aβ) plaques. In addition to the toxic properties of Aβ, the positive features of this peptide are increasingly being reported. At the clinical/preclinical study stage, the negative effect of compounds with anti-amyloidogenic activity was demonstrated. The phenomenon of Aβ aggregation is a physiological response that shows a strong antimicrobial activity [1,2,3], by which it protects the brain against the effects of harmful molecules penetrating the blood‑brain barrier, by which it combines bacterial fragments and forms insoluble pods with them [2, 4]. Moreover, there is a relationship between the number of bacteria in the types most frequently represented among patients and AD biomarkers (including the level of Aβ42/Aβ40 in the cerebrospinal fluid) [5]. A small-group study in Japan showed that reduced numbers of Bacteroides and increased numbers of bacteria labeled “other” are better indicators of AD than traditional biomarkers such as ApoEε4 or a high result of VSRAD test (voxel-based specific regional analysis system for Alzheimer’s disease) [6]. Inflammation, more specifically neuroinflammation, is a factor that can link infections, changes in the microbiota, particles secreted and produced by microorganisms, and changes in the brain leading to the development of AD symptoms [7, 8]. Astrocytes, microglia, and pro-inflammatory cytokines have been observed in the vicinity of amyloid plaques and neurofibrillary tangles for a relatively long time—Alzheimer himself is the first to mention the inflammatory component of the disease image [7,8,9].
The post-mortem material of patients with AD revealed bacterial origin particles, including fragments of bacterial cells (peptidoglycan, flagellin, lipopolysaccharide—LPS) and bacterial amyloid [10]. The most important bacterial amyloid curli fimbriae are produced by Escherichia coli (they are biofilm components, biochemically similar to Aβ). It has been suggested that, due to the similar bacterial structure, amyloids can induce human proteins to adopt the pathological structure of the β-sheet [11]. LPS is a strong pro-inflammatory agent, and together with amyloid can co-create senile plaques [11,12,13,14,15]. At the same time, it causes cell damage and death in a dose-dependent manner. By interacting with the toll-like 4 receptor (TLR4), LPS activates the NF-κB, p38MAPK, or JNK signaling pathways, resulting in subsequent inflammatory cell damage. Therefore, this particular bacterial cell outer membrane compound is widely used to induce inflammation in neuronal cells in in vitro models of spinal cord injury (SCI) or various neurodegenerative diseases, including AD [16, 17]. It has been proved in many experiments that PC12 cells treated with LPS showed increased production of pro-inflammatory cytokines such as Il-1β, Il-6, and TNF-α, as confirmed by mRNA expression measurements [18,19,20,21,22]. It is generally recognized that the amount of microglia and amyloid is dependent on each other based on feedback. When the amount of Aβ aggregates increases, they can stimulate toll-like receptors (TLRs) and enhance the inflammatory response [23, 24]. After TLR2 and TLR4 activation, glial cells and astrocytes release pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-12, IL-18) and anti-inflammatory cytokines (IL-10 and TGF-β) [25, 26]. The presence of Aβ aggregates and damaged and dead cells in the brain tissue permanently stimulates proliferation and activates microglial cells, which maintains the stimulation of non-specific immune response, which affects the release of chronic inflammatory reaction mediators and intensification of the production of reactive oxygen and nitrogen species (ROS and RNS). The persistent stimulation of non-specific immune response is considered a key mechanism is driving the progression of neurodegeneration [24, 27, 28].
PC12 is a cancer cell line derived from the rat adrenal glands, which, after incubation with nerve growth factor (NGF), differentiates into cells that resemble biochemically and phenotypically sympathetic nerves [29]. The human THP-1 cell line is derived from a human with acute monocytic leukemia. THP-1 cell cultures incubated in the presence of phorbol 12-myristate 13-acetate (PMA) differentiate into microglia-like cells [30]. THP-1 cells treated with PMA are—in terms of morphology, expression of characteristic surface proteins, production of cytokines, and response to various stimuli—similar or even identical to human macrophages. Therefore, THP-1 cells present a suitable macrophage-like model, especially in response to TLR2 agonists (like LPS or amyloid-β) [31]. Expression of the M-phenotype activation markers, namely the pro-inflammatory cytokines Il-1β and TNF-α, corresponds to the expression observed in human monocyte-derived macrophage cultures [32,33,34]. THP-1 induced to differentiation with PMA, after contact with bacterial lipopolysaccharide, also secretes—among other eicosanoid derivatives—PGE2 (prostaglandin E2), which leads to suppression of M2-type polarization and enhanced expression of key markers of M1-phenotype (mainly Il-1β) [33]. The used concentration of 5 ng/ml and overall PMA treatment protocol in other studies proved to be sufficient to induce differentiation, ensuring CD14 expression and response to even weak pro-inflammatory stimuli [31, 35]. At the same time, the relatively low concentration of PMA used in this experiment ensured minimal cell death rate [30]. Both cell lines (PC12 and THP-1) are widely used as models in neurobiological research.
This study aimed to determine whether the presence of low doses of Aβ has a protective effect in cultures of neuron-like and microglia-like cells preincubated with LPS.
Materials and Methods
Cell Lines and Conditions
The study was carried out on two cell lines, often used as an in vitro model in neurobiological research. The first cell line was the PC12 line growing in suspension, derived from rat adrenal pheochromocytoma. The second cell line was the THP-1, human monocytic cells obtained from an acute monocytic leukemia patient. Both cell lines were purchased from ATCC. These cell lines were cultured with RPMI-1640. In the case of PC12 cells, the medium was supplemented with 10% donor horse serum (DHS; EuroClone, Italy), 5% fetal bovine serum (FBS; Biological Industries, USA) with 2 mME L-glutamine and 25 μg/ml gentamicin. In contrast, for THP-1 cells, the same L-glutamine and gentamicin concentrations and only 10% FBS were applied. These media were used for standard culture. Cell morphology and confluence were assessed using a microscope at least twice a week. The medium with cells was collected into previously prepared and described centrifuge tubes and centrifuged at 1000×g for 5 min. Then, the supernatant was removed, and fresh medium was added. THP-1 cells were pipetted and counted with a Bürcker chamber. However, the pellet of PC12 cells had to be broken with a syringe with a needle (twice each needle with a diameter of 12, 9, and 6) and then also counted in the Bürcker chamber. For the cell lines used to constitute an appropriate neurobiological model, it was necessary to differentiate them. For this purpose, 1 day after plating the cells, the primary medium was replaced with the differentiation medium. The differentiation medium for PC12 cells contained 100 ng/ml nerve growth factor (NGF) and for THP-1 cells 50 ng/ml phorbol 12-myristate 13-acetate (PMA). PC12 cells were differentiated for 3 days, and THP-1 cells for 5 days, with a medium replacement every 2 days. Cultures were incubated at 5% CO2, 95% humidity, and 37 °C. The tests used differentiation media supplemented similarly with L-glutamine and gentamicin, but the serum was reduced to 1% DHS for PC12 cells and 1% FBS for THP-1 cells.
To analyze the neural characteristics of PC12 cells, immunocytofluorescence was performed. For this purpose, the doublecortin (DCX) antibody was used. After incubation with NGF, cells were fixed with 100% cold methanol for 5 min. The cells were then washed three times with PBST (0.1% Tween 20 with PBS) for 5 min. Permeabilization was performed with 0.1% Triton X-100 in PBS for 10 min at RT. The blocking of non-specific antibodies was then carried out with a PBST solution supplemented with 1% bovine serum albumin (BSA) and 10% normal goat serum for 30 min. The antibody was prepared by diluting 1:500 in 1% BSA in PBST. The culture plates were washed three times with PBST for 5 min, and finally, the cells were treated with the antibody solution for 1 h at room temperature. The plates were washed again and observed under a fluorescence microscope (EVOS FL, Thermo Fisher Scientific).
Tested Compounds
Lipopolysaccharide (LPS) (cat. no. L2630) from Escherichia coli and amyloid-β (25–35) (Aβ25–35) (cat. no. A4559) was purchased from Sigma-Aldrich, Saint Louis, USA. Both compounds were dissolved in distilled water to a final stock concentration of 1.0 mM. The stock solutions were stored at − 20 °C until use, but not longer than 6 months. For the biological experiments, the LPS was dissolved in the primary medium to a concentration range of 1–100 μM. The Aβ was also diluted at the primary medium but to concentrations of 0.001 μM and 1.0 μM.
Modification of the Surface of Culture Plates
PC12 cells grow in suspension, so to be a neural model, they must be stimulated to adhere to a culture vessel surface. For this purpose, the surface of the culture plates was modified, and the wells were covered with a type I collagen solution (Sigma-Aldrich). The purchased collagen was dissolved in 0.1 M acetic acid to the concentration of 0.1% (w/v) and stored at − 20 °C until the end, but not longer than 6 months. To obtain a working collagen solution, the stock solution was diluted with distilled water to a final concentration of 0.01% (w/v). The volume of the working solution necessary to cover the entire surface was pipetted into the well. The plates were left at 4–8 °C overnight. The following day, the remaining solution was removed, and the covered plates were washed three times for 5 min with PBS. The prepared plates were stored at − 4 °C for no longer than a month. Plates were UV irritated for 30 min before use.
Experiment Design
After the cells were differentiated, the medium was removed, and the LPS prepared in advance was added for 24 h at concentrations (1–100 μM). The next day the LPS solutions were removed, the culture was washed. The previously prepared Aβ was added at a concentration of 1.0 μM or 0.001 μM for the next 24 h in viability and genotoxicity assays or 1 h when the ROS level was measured. After this time, tests were performed to assess the viability of cell cultures, the level of free oxygen radicals, and DNA damage.
Viability Assays
Viability for both cell lines was measured by the MTT assay. Besides, an additional test was performed for PC12 cells by measuring LDH (lactate dehydrogenase) release. These assays were performed at a cell density of 10,000 per well. After incubation of PC12 cells with Aβ, the supernatant was transferred to new culture plates. To carry out the LDH assay, the reaction mixture was added to the previously collected supernatants and left for 30 min in the dark. After this time, the stop solution was added, and the absorbance was measured at two lengths, 490 nm and 680 nm, using a Victor2 multi-plate reader. To perform the MTT assay, the cells of both lines were washed, and 1 mg/ml MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) solution in MEM without phenol red was added into the culture. The cells were incubated for 2 h at 37 °C. The solution was then carefully removed, isopropanol was added, and plates were shaken for 30 min to dissolve the formazan crystals. Finally, the absorbance was measured at 555 nm using a Victor2.
ROS Level
The ROS level was measured with DCF-DA assay. Cells were seeded at a density of 20,000 cells/well. After 1-h incubation with Aβ, the solution was removed and added a 20 μM DCF-DA (2′-7′-dichlorofluorescin diacetate) solution for the next 1 h at 37 °C. After removal of the supernatant, the cells were washed with PBS, and the fluorescence was measured at with excitation at 485 nm and emission at 535 nm using a Victor2.
Fast Halo Assay
To assess the effect of Aβ on DNA damage in LPS-induced inflammation, the fast halo assay (FHA) was performed. The test was performed at a cell density of 50,000 per well. After incubation with Aβ, the supernatant was collected into pre-prepared tubes, and the culture was washed with PBS and harvested, and the TrypLE solution was added to the plates for 2 min at 37 °C. After collection of cell suspension, the tubes were centrifuged at 1000×g for 5 min. The supernatant was removed, and the pellet was resuspended in PBS and again centrifuged under the same conditions. The supernatant was removed again, and the pellet was resuspended to obtain a cell density of 30,000 in 30 μL DPBS and the tubes with cells were placed in a water bath at 37 °C. The cell suspension was then mixed with the agarose solution (1.25% low melting agarose in PBS), and the cell mixture was immediately pipetted between an agarose coated slide and a coverslip. This preparation was put on a cooling block and left to gel formation. The coverslip was removed, and preparation was put in lysis buffer overnight at 4 °C. The following day, the slides were moved in pH 13 buffer for 30 min at RT. After this time, the slides were transferred to a neutralization buffer twice for 5 min. Finally, the specimen was stained with 10 mM DAPI solution, and pictures were taken of 50 randomly encountered nuclei in each analyzed slide.
Neuronal Features
To evaluate neuronal features, the length of the neurites was measured. Only cells whose neurite length was twice the body diameter were assessed. Additionally, the number of neurites for each cell was counted. Calculations were performed for 100 randomly selected cells (from numerous micrographs taken) in 5 independent experiments. The ImageJ image analysis software was used to count and measure neurites on microscopic images. Those neurites for which it was certain which the cell neurite originated and where it ends were assessed.
Statistical Analysis
The results are presented as mean ± SEM. In this study, two control groups were used: negative control was cultured only primary medium without LPS, and Aβ and positive control were grown only with LPS without Aβ. The distribution of the results was normal, and the equality of variance was confirmed with Levene’s test, so statistical significance was calculated using parametric tests—ANOVA and the appropriate post hoc test. The p < 0.05 was considered the significance point. The Statistica v13.1 software was used for statistical analysis.
Results
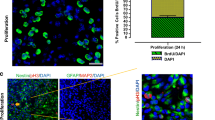
Immunocytofluorescence staining was performed to check whether the cultures show neuron-like features after 72-h incubation of PC12 cells with NGF. For this purpose, PE-conjugated doublecortin (DCX) was used. The doublecortin protein is characteristic of young developing neurons [29, 36, 37]. After staining, it was shown that PC12 cells after 72 h of incubation with NGF-containing medium express doublecortin protein (Fig. 1a). Figure 1b shows the culture of THP-1 cells after incubation with PMA for 5 days. As a result of the incubation of the monocyte line in the medium containing PMA, the cell morphology changed—from cell suspension to cells adhering to culture vessels’ surface. Simultaneously, a change in the cells’ shape was observed—from spherical, almost identical cells, the culture became heterogeneous—flattened and round cells and cells with a significantly increased body size were observed along with the reorganization of the cytoskeleton and the development of branches, which constituted about 80% of cells in the field of view [38].
To investigate the effect of Aβ on the viability of two cell lines: PC12 after differentiation from nerve growth factor (NGF) and THP-1 after PMA treatment after prior LPS exposure, metabolic activity (MTT assay) was assessed (Fig. 2a and b). A statistically significant decrease in metabolic activity after incubation with LPS was observed in both PC12 and THP-1 cells (except 1 μM in PC12 cells). A concentration dependence was observed in the PC12 cell line. However, in the THP-1 line, regardless of the concentration used, a decrease in viability by about 20% was observed. Both 0.001 μM and 1.0 μM concentrations of Aβ influence the reduction of negative impact LPS on both cell lines.
Effect of Aβ on PC12 cells (a and c) and THP-1 (b) cells preincubated with lipopolysaccharide (LPS); metabolic activity measured in MTT assay (a and b), cell viability measured in LDH assay (c); control—cell culture incubated without LPS and Aβ; * p < 0.05—significant difference compared to a negative control without LPS; # p < 0.05—significant difference compared to control preincubated with LPS
The level of lactate dehydrogenase (LDH) release was also assessed, which indirectly indicates the number of necrotic cells in culture after incubation with LPS and then with Aβ. After PC12 cells were incubated in the presence of LPS, a concentration-dependent increase in the amount of released lactate dehydrogenase was observed. A statistically significant reduction in lactate dehydrogenase leakage was observed after incubation with Aβ. The regenerative effect of the 1.0 μM Aβ was significantly strong (reduction of LDH leakage was observed) even compared to the negative control (PC12 culture only on LPS and Aβ free medium) after preincubating the culture in the range 1–50 μM LPS. In contrast, a similar LDH leakage reduction effect (about 20%) compared to the negative control was seen with 0.001 Aβ only at 1.0 μM LPS.
To assess intracellular ROS, accumulation was measured with the DCF-DA assay (Fig. 3a and b). This study demonstrated a higher level of reactive oxygen species in PC12 cells than in THP-1 cells after LPS treatment. In both cell lines, an increase in ROS dependent on LPS concentration was observed. After applying the concentration of 0.001 and 1.0 μM Aβ, the peptide’s regenerative effect was observed. That is, it reduced the level of free oxygen radicals (statistically significant). In both lines (PC12 and THP-1) after incubation with Aβ, regardless of the concentration used, a reduction in free oxygen radicals was observed to the level seen in the negative control after prior incubation with 100 μM LPS. A similar effect was demonstrated in the THP-1 line after incubation with a concentration of 50 μM LPS and PC12 cell line after treatment with a concentration of 1 μM LPS. In other cases, a statistically significant reduction in the level of free oxygen radicals was observed compared to the negative control.
Effect of Aβ on PC12 cells (a) and THP-1 (b) cells preincubated with lipopolysaccharide (LPS); (a and b) DCF-DA assay; control—cell culture incubated without LPS and Aβ; * p < 0.05—significant difference compared to a negative control without LPS; # p < 0.05—significant difference compared to control preincubated with LPS
It is well known that high levels of ROS can lead to double-strand break (DSB). Accordingly, a fast halo assay (FHA) was performed, which measured the nuclear size of the halo (chromatin dispersion). In LPS-induced cells, the number of DSBs was almost twice and four times higher compared to the negative control at the highest concentration tested (100 μM) in the culture of THP-1 and PC12 cells, respectively (Fig. 4a and b). Aβ in concentrations of 0.001 and 1.0 showed a regenerative effect on damage to DNA strand breaks after prior incubation with LPS.
Effect of Aβ on PC12 cells (a) and THP-1 (b) cells preincubated with lipopolysaccharide (LPS); (a and b) fast halo assay; control—cell culture incubated without LPS and Aβ; * p < 0.05—significant difference compared to a negative control without LPS; # p < 0.05—significant difference compared to control preincubated with LPS
To evaluate the impact on the neuronal feature of PC12 cells, the average length of neurites (Fig. 5a) and the average number of neurites (Fig. 5b) were analyzed. Incubation with LPS caused a statistically significant decrease in neurite length (to up 60% in higher concentration). For all concentrations of Aβ, an increase in the average length of measured neurites was shown compared to LPS-induced cells. The neurite growth was statistically significantly greater after applying Aβ at a concentration of 0.001 μM than at 1.0 μM. At the same time, this increase was comparable to the negative control.
Effect of Aβ on PC12 cells preincubated with lipopolysaccharide (LPS); average length of neurites (a), an average number of neurites (b); control—cell culture incubated without LPS and Aβ; * p < 0.05—significant difference compared to a negative control without LPS; # p < 0.05—significant difference compared to control preincubated with LPS
The average number of neurites after incubation of cells with LPS was statistically significantly lower in the concentration range of 5–100 μM compared to the negative control. Moreover, the reduction in the number of neurites was concentration-dependent. An increase in the mean number of neurites was demonstrated after incubating the culture with Aβ at both 0.001 and 1.0 μM concentrations. The average neurite length was 40 μm for negative control. It is worth noting that after incubation with Aβ at a concentration of 0.001 μM, a neurotrophic activity could be observed, and the average length of neurites was about 58 μm (Fig. 5a). Figure 6 shows exemplary micrographs showing the morphology of PC12 cells. The longest neurites are in Fig. 6e (1.0 μM LPS and 0.001 μM Aβ), and the culture shown in Fig. 6d (100.0 μM LPS without Aβ) is characterized by the shortest neurites.
Effect of Aβ on PC12 cells preincubated with lipopolysaccharide (LPS); incubation without Aβ (a‑d), with Aβ at a concentration of 0.001 μM (e‑h), and with Aβ at a concentration of 1 μM (i‑l); LPS at a concentration of 1.0 μM (a, e, i), 5.0 μM (b, f, j), 50.0 μM (c, g, k), and 100.0 μM (d, h, l); objective magnification: × 10
Discussion
A growing body of evidence suggests that amyloid-β—considered for a long time to be a hallmark for pathology with no physiological function—may have antimicrobial properties as a part of the innate immune system. Aβ molecules share some striking similarities with recently recognized antimicrobial peptides (AMPs)—potent antibiotics with immunomodulatory properties produced in human bodies—together with its most thoroughly studied representative LL-37 protein. The tendency to self-assemble to form oligomers in pathology may lead to creating senile plaques typical for Alzheimer’s disease. Still, it has been proven that Aβ oligomerization enables binding, agglutination, and entrapment of certain pathogens while inhibiting their adhesion preventing the spread of infection and facilitating phagocytosis [1, 2, 39,40,41]. Aβ molecular structure, just like LL-37, contains a heparin-binding motif enabling attachment to carbohydrates being a part of the microbial cell wall [2, 42]. As studies show, Aβ can stop microbes from spreading and may kill viruses, fungi, and bacteria. Microbicidal properties of known AMPs usually stem from their capability to self-aggregate in the form of fibrils and interacting with negatively charged microbial plasma membranes (and sometimes mitochondria in line with the endosymbiotic theory of their origin), leading to uncontrolled ion leakage and death of attacked cell [4, 42, 43].
Numerous studies report that circulating concentrations of amyloid-β (including neurotoxic oligomers) vary immensely between AD patients [44], probably due to various measuring methods and inconsistency in the preanalytical treatment of specimens. It is already established that plasma levels of amyloid-β correlate poorly with patients’ clinical conditions [45]. Therefore in our study, we chose concentrations of soluble amyloid-β observed in brain homogenates and CSF (cerebrospinal fluid) of AD subjects that are ranging from nanomoles [46, 47] to micromoles [47,48,49,50]. Concentrations of 1 nM and 1 μM, respectively, were selected due to the neurotrophic properties of nanomolar concentrations of Aβ25–35 [51] and reported (dose-dependent) neurotoxicity of micromolar concentrations [52, 53].
As early as the 1990s, scientists tried to draw attention to the positive effects of Aβ in cell cultures. Luo et al. proved that in the culture of PC12 cells, Aβ at a concentration of 1 nM had a pro-proliferative effect (even stronger than BSA administration). Moreover, Aβ25–35 has been shown to have a stronger pro-proliferative activity compared to the two more frequently studied Aβ fragments, 1–40 and 1–42 [54]. The next step in demonstrating the positive effect of Aβ on cell cultures was to evaluate its effect on PC12 cells during differentiation. Yanker et al. observed that during the first 48 h of differentiation, the presence of Aβ1–40 showed a neurotrophic activity, while in the following days, it was neurotoxic. At the same time, Aβ25–35 was shown to have a stronger neurotrophic effect compared to other fragments, and the impact on the viability was not so pronounced [51]. The neurotrophic effect of Aβ1–42 (even at a concentration of 1.0 μM) was also demonstrated in the cultures of neural stem cells [55]. An interesting observation was also made by Arevalo et al., who conducted in vitro studies on neurons isolated from the mouse hippocampus. They added to the cell culture Aβ at a concentration of 20 nM and 800 nM. They found that the low concentration had a positive effect on the length of neurites but reduced the density. The opposite effect was observed at a higher concentration (800 nM) [56]. In our study, it was shown that amyloid in low concentrations has a regenerative effect on neuron-like cells (PC12) and influenced the viability and growth as well as the number of neurites in the culture after prior incubation of the culture with LPS. It has also been shown that physiologically low concentrations of Aβ have neurotrophic properties. We noticed that a low concentration of Aβ (0.001 μM) had a strong neurotrophic effect on neurite length, while the effect on neurite density was comparable to the control (without LPS).
Reactive oxygen species production catalyzed by bound metal (zinc or copper) and activating complement are also mediated by oligomers formation [39]. As a member of the AMP group, Aβ is an agent of the innate immune response in the immune-privileged brain, and its expression is heightened not only in AD but also in neuroborreliosis, neurosyphilis, HIV-related dementia, herpes simplex encephalitis, and Chlamydia brain infection [1, 40, 41, 57]. Further proving Aβ peptide’s engagement in immune response in the brain, apolipoprotein E is suggested to be a part of innate immunity and its homozygous variant ε4—the major genetic risk factor for sporadic Alzheimer’s disease—is associated with increased susceptibility to some pathogenic microbes including neurotropic viruses [39, 41]. Additionally, the brains of patients with AD tend to show higher viral or bacterial loads than their age-matched controls. A large proportion of Aβ-formed plaques contains particles and genetic material of viruses and bacteria [41]. We proved that fragments of bacterial cells, in this case, LPS, caused an increase in the level of free oxygen radicals after 24-h incubation of PC12 cultures with LPS. In contrast, the incubation of PC12 cell cultures with Aβ caused the scavenging of free radicals. At the same time, along with a decrease in the level of free oxygen radicals, the regeneration of DNA strand damage was observed.
Antimicrobial properties of Aβ, either synthetically produced or obtained from the brain and temporal lobe homogenates of AD patients and Aβ-overexpressing transgenic animals, have been proven in vitro for a variety of pathogenic microbes, including Gram-positive and Gram-negative bacteria, viruses like influenza A and herpes simplex virus type 1, and also Candida albicans [1, 4, 39, 41]. Aβ1–42 showed higher than Aβ1–40 antimicrobial activity and the ability to agglutinate pathogens, which might be connected to hydrophobicity and readiness to self-aggregate Aβ1–42. Observed effects were obliterated after treatment with anti-amyloid beta antibodies [1, 2, 40, 43]. In vivo experiments revealed a higher survival rate of Aβ-overexpressing 5xFAD mice infected intracerebrally with Salmonella Typhimurium with lower cerebral bacterial loads than their non-transgenic littermates [2, 39, 41]. Similarly, transgenic nematodes Caenorhabditis elegans producing Aβ1–42 isoform (GMC101) showed reduced mortality after C. albicans infection than control CL2122 worms [2, 39, 41].
Treatments targeted at amyloid-β oligomers and fibrils perceived then as purely pathological structures showed numerous side effects in clinical trials, including a higher risk of meningitis and upper respiratory tract infections. They increased blood‑brain barrier permeability leading to edema, micro-hemorrhages, and neurovascular disturbances [1, 39, 41, 42]. Adverse effects of anti-Aβ therapy seem to be directly or indirectly related to disturbed physiological functions of said peptide.
Aβ senile plaques, which may also contain other proteins and fragments of bacterial cells, including LPS, cannot be effectively removed by microglial cells. These deposits strongly activate pro-inflammatory signaling pathways in brain tissue cells (including cytokines) and increase the production of reactive oxygen and nitrogen species [25]. The role of the activation of the non-specific immune response pathway in the development of neurodegeneration may be an attractive target for future therapy because post-mortem studies of brain tissue in people with mild cognitive impairment (MCI) showed a significant increase in the number of activated microglial cells (immunophenotype M2) and a significant increase in the activity of the NLRP3-caspase complex 1 [23]. An important mechanism driving the progress of neurodegenerative processes is the persistent inflammation caused by the activation of a non-specific immune response leading to the release of pro-inflammatory cytokines and the maintenance of neuronal oxidative stress. The presence of Aβ oligomers inside cells, extracellular deposits of Aβ aggregates in senile plaques, and the presence of damaged and dead cells in brain tissue can induce a non-specific immune response in Alzheimer’s disease. The neuroprotective task of astrocytes and microglia is the removal of apoptotic and necrotic cells and amyloid aggregates from the brain tissue [58, 59]. However, excessive proliferation of glial cells and their activation can exacerbate the inflammatory process, damage neurons, and affect the loss of synaptic spines, thereby reducing synaptic function [58,59,60]. At the same time, the multiplication and activation of microglia cells are associated with the constant presence of Aβ aggregates and damaged and dead cells in the brain tissue, which affects the increased production of ROS and reactive forms of nitrogen (RNS) and the release of chronic inflammatory mediators. Long-term stimulation of non-specific immune response is believed to be a key mechanism driving neurodegeneration progression [24, 27, 28]. In connection with the “vicious circle” between Aβ level and microglia amount, we wanted to see how bacterial particle (LPS) fragments affect microglia cells and then whether the physiological Aβ concentration would have a regenerative effect on LPS damage. The study showed that LPS increased the level of free oxygen radicals and damaged DNA strands in microglia cells. At the same time, incubation with Aβ at physiological concentrations caused less damage to both the DNA strand and the ROS level than after incubation with LPS. The incubation of microglia with LPS caused a decrease in cells’ metabolic activity, while incubation with Aβ resulted in activity similar to the negative control. This may indicate a persistent balance between the Aβ and the microglia. The presence of amyloid reduced the level of ROS, similar to the negative control. Only the regeneration of DNA damage was weaker. The likely mechanism of action of Aβ on nerve cells and microglia is shown in Fig. 7.
The condition of the patient’s microbiota may be of great importance in Alzheimer’s disease. The variety of microorganisms plays a significant role in digestion and providing the host with nutrients. However, if these organisms, their fragments, or substances produced by them cross the intestinal epithelium’s protective barrier, it can pose a serious threat. Microorganisms do not even have to leave the gastrointestinal tract to influence metabolism, immune response, and even host behavior, which may be related to the “microbiota-gut-brain axis”. The intestinal microbiota contacts the central nervous system through a complex network of signals consisting of neurotransmitters, conduction of stimuli through the vagus nerve and the autonomic nervous system, and secreted short-chain fatty acids (SCFAs), micro RNA (miRNA), small non-coding (sncRNA), and other active molecules, as well as changes in the permeability of the blood‑brain barrier (BBB) [14, 61,62,63]. Our study demonstrated the toxic effect of bacterial LPS on neuron-like and microglia-like cells, as well as the regenerative effect of low doses of Aβ. This is another step that challenges the amyloid hypothesis. It seems likely that high Aβ levels are only the brain’s response to an excess of harmful factors (including LPS) that lead to neuroinflammation.
This work’s limitation is the lack of analysis of the influence of co-cultures of neuronal cells and microglia. The authors plan to conduct such research in the next stage of work. It is also planned to determine the level of pro-inflammatory cytokines in cultures. Moreover, the concept of this study was based on the hypothesis of amyloid cascade and neuroinflammation as the main pathomechanism involved in Alzheimer’s disease and especially on the idea that amyloid-β, as a member of AMPs (antimicrobial proteins), could have a beneficial effect on neuronal cells in the presence of bacterial LPS and that disturbance in the physiological pathways involving Aβ and causing neuroinflammation might be the underlying cause of Alzheimer’s disease development [64,65,66]. However, emerging new studies suggest that a model based on tau protein pathology should also be considered in terms of the development and propagation of the disease. Tau burden correlates with clinical symptoms and is needed in some in vitro studies or animal models of AD for the appearance of neuronal damage [64, 65, 67, 68]. Therefore, the inclusion of tau protein in future research, in addition to amyloid-β, could result in a new and better understanding of the mechanisms leading to AD pathology, especially as there are still few experiments considering tau hyperphosphorylation as more than just an Aβ-induced phenomenon [68,69,70,71].
In conclusion, LPS in the entire range of concentrations tested increased ROS levels and frequency of DNA damage in THP-1 and PC12 cultures and did not affect neurite growth in PC12 cells. In contrast, Aβ at low concentrations [0,001–0.1 μM] did not increase toxicity, DNA damage, and ROS levels in THP-1 and PC12 cultures, while significantly increased neurite growth in PC12 cultures. Further research is needed to confirm the neuroprotective effect of these low (close to physiological) Aβ concentrations.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, Burton MA, Goldstein LE et al (2010) The Alzheimer’s disease-associated amyloid β-protein is an antimicrobial peptide. PLoS One 5:e9505. https://doi.org/10.1371/journal.pone.0009505
Kumar DKV, Choi SH, Washicosky KJ et al (2016) Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med 8:340ra72. https://doi.org/10.1126/scitranslmed.aaf1059
Schwartz K, Boles BR (2013) Microbial amyloids-functions and interactions within the host. Curr Opin Microbiol 16:93–99. https://doi.org/10.1016/j.mib.2012.12.001
Brothers HM, Gosztyla ML, Robinson SR (2018) The physiological roles of amyloid-β peptide hint at new ways to treat Alzheimer’s disease. Front Aging Neurosci 10:118. https://doi.org/10.3389/fnagi.2018.00118
Vogt NM, Kerby RL, Dill-McFarland KA et al (2017) Gut microbiome alterations in Alzheimer’s disease. Sci Rep 7:13537. https://doi.org/10.1038/s41598-017-13601-y
Saji N, Niida S, Murotani K, Hisada T, Tsuduki T, Sugimoto T, Kimura A, Toba K et al (2019) Analysis of the relationship between the gut microbiome and dementia: a cross-sectional study conducted in Japan. Sci Rep 9:1008. https://doi.org/10.1038/s41598-018-38218-7
Alkasir R, Li J, Li X, Jin M, Zhu B (2017) Human gut microbiota: the links with dementia development. Protein Cell 8:90–102. https://doi.org/10.1007/s13238-016-0338-6
Minter MR, Taylor JM, Crack PJ (2016) The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease. J Neurochem 136:457–474. https://doi.org/10.1111/jnc.13411
Pimplikar SW (2014) Neuroinflammation in Alzheimer’s disease: from pathogenesis to a therapeutic target. J Clin Immunol 34:64–69. https://doi.org/10.1007/s10875-014-0032-5
Sobol CV (2018) Role of microbiota in neurodegenerative diseases. Russ J Dev Biol 49:297–313. https://doi.org/10.1134/S1062360418060061
Kowalski K, Mulak A (2019) Brain-gut-microbiota axis in Alzheimer’s disease. J Neurogastroenterol Motil 25:48–60. https://doi.org/10.5056/jnm18087
Sochocka M, Donskow-Łysoniewska K, Diniz BS, Kurpas D, Brzozowska E, Leszek J (2019) The gut microbiome alterations and inflammation-driven pathogenesis of Alzheimer’s disease—a critical review. Mol Neurobiol 56:1841–1851. https://doi.org/10.1007/s12035-018-1188-4
Zhan X, Stamova B, Sharp FR (2018) Lipopolysaccharide associates with amyloid plaques, neurons and oligodendrocytes in Alzheimer’s disease brain: a review. Front Aging Neurosci 10:42. https://doi.org/10.3389/fnagi.2018.00042
Zhao Y, Cong L, Lukiw WJ (2017) Lipopolysaccharide (LPS) accumulates in neocortical neurons of Alzheimer’s disease (AD) brain and impairs transcription in human neuronal-glial primary co-cultures. Front Aging Neurosci 9:407. https://doi.org/10.3389/fnagi.2017.00407
Zhan X, Stamova B, Jin L-W, DeCarli C, Phinney B, Sharp FR (2016) Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology 87:2324–2332. https://doi.org/10.1212/WNL.0000000000003391
Park BS, Lee J-O (2013) Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med 45:e66. https://doi.org/10.1038/emm.2013.97
Batista CRA, Gomes GF, Candelario-Jalil E, Fiebich BL, de Oliveira ACP (2019) Lipopolysaccharide-induced neuroinflammation as a bridge to understand neurodegeneration. Int J Mol Sci 20:2293. https://doi.org/10.3390/ijms20092293
Zhang G, Liu Y, Xu L, Sha C, Zhang H, Xu W (2019) Resveratrol alleviates lipopolysaccharide-induced inflammation in PC-12 cells and in rat model. BMC Biotechnol 19:10. https://doi.org/10.1186/s12896-019-0502-1
Li R, Yin F, Guo Y, Ruan Q, Zhu Q (2018) Angelica polysaccharide protects PC-12 cells from lipopolysaccharide-induced injury via down-regulating microRNA-223. Biomed Pharmacother 108:1320–1327. https://doi.org/10.1016/j.biopha.2018.09.147
Sun Y, Liu B, Zheng X, Wang D (2019) Notoginsenoside R1 alleviates lipopolysaccharide-triggered PC-12 inflammatory damage via elevating microRNA-132. Artif Cells Nanomed Biotechnol 47:1808–1814. https://doi.org/10.1080/21691401.2019.1610414
Ma S, Zhang C, Zhang Z, Dai Y, Gu R, Jiang R (2019) Geniposide protects PC12 cells from lipopolysaccharide-evoked inflammatory injury via up-regulation of miR-145-5p. Artif Cells Nanomed Biotechnol 47:2875–2881. https://doi.org/10.1080/21691401.2019.1626406
Jiang J, Wang G (2020) Matrine protects PC12 cells from lipopolysaccharide-evoked inflammatory injury via upregulation of miR-9. Pharm Biol 58:314–320. https://doi.org/10.1080/13880209.2020.1719165
Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D et al (2013) NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493:674–678. https://doi.org/10.1038/nature11729
Gąsiorowski K, Brokos B, Echeverria V, Barreto GE, Leszek J (2018) RAGE-TLR crosstalk sustains chronic inflammation in neurodegeneration. Mol Neurobiol 55:1463–1476. https://doi.org/10.1007/s12035-017-0419-4
Piirainen S, Youssef A, Song C, Kalueff AV, Landreth GE, Malm T, Tian L (2017) Psychosocial stress on neuroinflammation and cognitive dysfunctions in Alzheimer’s diseasethe emerging role for microglia? Neurosci Biobehav Rev 77:148–164. https://doi.org/10.1016/j.neubiorev.2017.01.046
Wang W-Y, Tan M-S, Yu J-T, Tan L (2015) Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med 3:136. https://doi.org/10.3978/j.issn.2305-5839.2015.03.49
Leszek J, Barreto GE, Gąsiorowski K et al (2016) Inflammatory mechanisms and oxidative stress as key factors responsible for progression of neurodegeneration: role of brain innate immune system. CNS Neurol Disord Drug Targets 15:329–336. https://doi.org/10.2174/1871527315666160202125914
Heneka MT, Golenbock DT, Latz E (2015) Innate immunity in Alzheimer’s disease. Nat Immunol 16:229–236. https://doi.org/10.1038/ni.3102
Wiatrak B, Kubis-Kubiak A, Piwowar A, Barg E (2020) PC12 cell line: cell types, coating of culture vessels, differentiation and other culture conditions. Cells 9:958. https://doi.org/10.3390/cells9040958
Starr T, Bauler TJ, Malik-Kale P, Steele-Mortimer O (2018) The phorbol 12-myristate-13-acetate differentiation protocol is critical to the interaction of THP-1 macrophages with Salmonella Typhimurium. PLoS One 13:e0193601. https://doi.org/10.1371/journal.pone.0193601
Lund ME, To J, O’Brien BA, Donnelly S (2016) The choice of phorbol 12-myristate 13-acetate differentiation protocol influences the response of THP-1 macrophages to a pro-inflammatory stimulus. J Immunol Methods 430:64–70. https://doi.org/10.1016/j.jim.2016.01.012
Daigneault M, Preston JA, Marriott HM, Whyte MKB, Dockrell DH (2010) The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One 5:e8668. https://doi.org/10.1371/journal.pone.0008668
Sheppe AEF, Kummari E, Walker A, Richards A, Hui WW, Lee JH, Mangum L, Borazjani A et al (2018) PGE2 augments inflammasome activation and M1 polarization in macrophages infected with Salmonella Typhimurium and Yersinia enterocolitica. Front Microbiol 9:2447. https://doi.org/10.3389/fmicb.2018.02447
Tedesco S, De Majo F, Kim J et al (2018) Convenience versus biological significance: are PMA-differentiated THP-1 cells a reliable substitute for blood-derived macrophages when studying in vitro polarization? Front Pharmacol 9:71. https://doi.org/10.3389/fphar.2018.00071
Park EK, Jung HS, Yang HI, Yoo MC, Kim C, Kim KS (2007) Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm Res 56:45–50. https://doi.org/10.1007/s00011-007-6115-5
Shmueli O, Gdalyahu A, Sorokina K, Nevo E, Avivi A, Reiner O (2001) DCX in PC12 cells: CREB-mediated transcription and neurite outgrowth. Hum Mol Genet 10:1061–1070. https://doi.org/10.1093/hmg/10.10.1061
Lee Y-W, Stachowiak EK, Birkaya B, Terranova C, Capacchietti M, Claus P, Aletta JM, Stachowiak MK (2013) NGF-induced cell differentiation and gene activation is mediated by integrative nuclear FGFR1 signaling (INFS). PLoS One 8:e68931. https://doi.org/10.1371/journal.pone.0068931
Grytting VS, Olderbø BP, Holme JA, Samuelsen JT, Solhaug A, Becher R, Bølling AK (2019) Di-n-butyl phthalate modifies PMA-induced macrophage differentiation of THP-1 monocytes via PPARγ. Toxicol in Vitro 54:168–177. https://doi.org/10.1016/j.tiv.2018.09.004
Moir RD, Lathe R, Tanzi RE (2018) The antimicrobial protection hypothesis of Alzheimer’s disease. Alzheimers Dement 14:1602–1614. https://doi.org/10.1016/j.jalz.2018.06.3040
Spitzer P, Condic M, Herrmann M, Oberstein TJ, Scharin-Mehlmann M, Gilbert DF, Friedrich O, Grömer T et al (2016) Amyloidogenic amyloid-β-peptide variants induce microbial agglutination and exert antimicrobial activity. Sci Rep 6:32228. https://doi.org/10.1038/srep32228
Gosztyla ML, Brothers HM, Robinson SR (2018) Alzheimer’s amyloid-β is an antimicrobial peptide: a review of the evidence. J Alzheimers Dis 62:1495–1506. https://doi.org/10.3233/JAD-171133
Welling MM, Nabuurs RJA, van der Weerd L (2015) Potential role of antimicrobial peptides in the early onset of Alzheimer’s disease. Alzheimers Dement 11:51–57. https://doi.org/10.1016/j.jalz.2013.12.020
Zhao Y, Lukiw WJ (2015) Microbiome-generated amyloid and potential impact on amyloidogenesis in Alzheimer’s disease (AD). J Nat Sci 1:e138
Hölttä M, Hansson O, Andreasson U, Hertze J, Minthon L, Nägga K, Andreasen N, Zetterberg H et al (2013) Evaluating amyloid-β oligomers in cerebrospinal fluid as a biomarker for Alzheimer’s disease. PLoS One 8:e66381. https://doi.org/10.1371/journal.pone.0066381
Chen C-C, Bates R, Carlson J (2014) Effect of environmental and cultural conditions on medium pH and explant growth performance of Douglas-fir ( Pseudotsuga menziesii) shoot cultures. F1000Research 3:298. https://doi.org/10.12688/f1000research.5919.2
Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, Lopresti BJ, Ziolko S et al (2008) Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain 131:1630–1645. https://doi.org/10.1093/brain/awn016
Peña F, Ordaz B, Balleza-Tapia H, Bernal-Pedraza R, Márquez-Ramos A, Carmona-Aparicio L, Giordano M (2010) Beta-amyloid protein (25-35) disrupts hippocampal network activity: role of Fyn-kinase. Hippocampus 20:78–96. https://doi.org/10.1002/hipo.20592
Bates KA, Verdile G, Li QX, Ames D, Hudson P, Masters CL, Martins RN (2009) Clearance mechanisms of Alzheimer’s amyloid-beta peptide: Implications for therapeutic design and diagnostic tests. Mol Psychiatry 14:469–486. https://doi.org/10.1038/mp.2008.96
Gregory GC, Halliday GM (2005) What is the dominant aβ species in human brain tissue? A review. Neurotox Res 7:29–41. https://doi.org/10.1007/BF03033774
Patton RL, Kalback WM, Esh CL, Kokjohn TA, van Vickle GD, Luehrs DC, Kuo YM, Lopez J et al (2006) Amyloid-β peptide remnants in AN-1792-immunized Alzheimer’s disease patients: a biochemical analysis. Am J Pathol 169:1048–1063. https://doi.org/10.2353/ajpath.2006.060269
Yankner B, Duffy L, Kirschner D (1990) Neurotrophic and neurotoxic effects of amyloid β protein: reversal by tachykinin neuropeptides. Science 250:279–282. https://doi.org/10.1126/science.2218531
Cuevas E, Rosas-Hernandez H, Burks SM, Ramirez-Lee MA, Guzman A, Imam SZ, Ali SF, Sarkar S (2019) Amyloid Beta 25–35 induces blood-brain barrier disruption in vitro. Metab Brain Dis 34:1365–1374. https://doi.org/10.1007/s11011-019-00447-8
Zheng X, Xie Z, Zhu Z, Liu Z, Wang Y, Wei LF, Yang H, Yang HN et al (2014) Methyllycaconitine alleviates amyloid-β peptides-induced cytotoxicity in SH-SY5Y cells. PLoS One 9:e111536. https://doi.org/10.1371/journal.pone.0111536
Luo Y, Sunderland T, Roth GS, Wolozin B (1996) Physiological levels of β-amyloid peptide promote PC12 cell proliferation. Neurosci Lett 217:125–128. https://doi.org/10.1016/0304-3940(96)13087-1
López-Toledano MA, Shelanski ML (2004) Neurogenic effect of β-amyloid peptide in the development of neural stem cells. J Neurosci 24:5439–5444. https://doi.org/10.1523/JNEUROSCI.0974-04.2004
Arevalo MÁ, Roldan PM, Chacón PJ, Rodríguez-Tebar A (2009) Amyloid β serves as an NGF-like neurotrophic factor or acts as a NGF antagonist depending on its concentration. J Neurochem 111:1425–1433. https://doi.org/10.1111/j.1471-4159.2009.06412.x
Clark IA, Vissel B (2015) Amyloid β: one of three danger-associated molecules that are secondary inducers of the proinflammatory cytokines that mediate Alzheimer’s disease. Br J Pharmacol 172:3714–3727. https://doi.org/10.1111/bph.13181
ElAli A, Rivest S (2016) Microglia in Alzheimer’s disease: a multifaceted relationship. Brain Behav Immun 55:138–150. https://doi.org/10.1016/j.bbi.2015.07.021
Hansen DV, Hanson JE, Sheng M (2018) Microglia in Alzheimer’s disease. J Cell Biol 217:459–472. https://doi.org/10.1083/jcb.201709069
Heppner FL, Ransohoff RM, Becher B (2015) Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 16:358–372. https://doi.org/10.1038/nrn3880
Foster JA, Rinaman L, Cryan JF (2017) Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress 7:124–136. https://doi.org/10.1016/J.YNSTR.2017.03.001
Lukiw WJ (2016) Bacteroides fragilis lipopolysaccharide and inflammatory signaling in Alzheimer’s disease. Front Microbiol 7:1544. https://doi.org/10.3389/fmicb.2016.01544
Sandhu KV, Sherwin E, Schellekens H, Stanton C, Dinan TG, Cryan JF (2017) Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res 179:223–244. https://doi.org/10.1016/J.TRSL.2016.10.002
Makin S (2018) The amyloid hypothesis on trial. Nature 559:S4–S7. https://doi.org/10.1038/d41586-018-05719-4
Paroni G, Bisceglia P, Seripa D (2019) Understanding the amyloid hypothesis in Alzheimer’s disease. J Alzheimers Dis 68:493–510. https://doi.org/10.3233/JAD-180802
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8:595–608. https://doi.org/10.15252/emmm.201606210
Kametani F, Hasegawa M (2018) Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s disease. Front Neurosci 12:25. https://doi.org/10.3389/fnins.2018.00025
Li Y, Xu P, Shan J, Sun W, Ji X, Chi T, Liu P, Zou L (2020) Interaction between hyperphosphorylated tau and pyroptosis in forskolin and streptozotocin induced AD models. Biomed Pharmacother 121:109618. https://doi.org/10.1016/j.biopha.2019.109618
Yao K, Zhao YF, Zu HB (2019) Melatonin receptor stimulation by agomelatine prevents Aβ-induced tau phosphorylation and oxidative damage in PC12 cells. Drug Des Devel Ther 13:387–396. https://doi.org/10.2147/DDDT.S182684
Zhou Y, Li C, Li D, Zheng Y, Wang J (2017) IL-5 blocks apoptosis and tau hyperphosphorylation induced by Aβ25–35 peptide in PC12 cells. J Physiol Biochem 73:259–266. https://doi.org/10.1007/s13105-017-0550-8
Cui Z, Tao T, Cheng C, Yang J, Shen Q, Ji Y, Li X, Liu H et al (2010) SSeCKS promote beta-amyloid-induced PC12 cells neurotoxicity by up-regulating tau phosphorylation in Alzheimer’s disease. Mol Cell Biochem 340:257–263. https://doi.org/10.1007/s11010-010-0425-6
Funding
The study was supported by funding from Wroclaw Medical University.
Author information
Authors and Affiliations
Contributions
Benita Wiatrak and Katarzyna Balon performed the in vitro experiments, collected data, and wrote the manuscript; Benita Wiatrak designed the study, prepared the figures, and analyzed the results. Both authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
This in vitro study did not include experiments on animals or human participants. The study used only commercially available cell lines from ATCC collection and no ethical approval is required.
Conflict of Interest
The authors declare that they have no conflict of interest.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wiatrak, B., Balon, K. Protective Activity of Aβ on Cell Cultures (PC12 and THP-1 after Differentiation) Preincubated with Lipopolysaccharide (LPS). Mol Neurobiol 58, 1453–1464 (2021). https://doi.org/10.1007/s12035-020-02204-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02204-w