Abstract
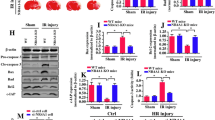
Hypoxia and reperfusion produces overproduction of ROS (reactive oxygen species), which may lead to mitochondrial dysfunction leading to cell death and apoptosis. Here, we explore the hypothesis that Prdx6 protects the spinal cord white matter from hypoxia-reperfusion injury and elucidate the possible mechanism by which Prdx6 elicits its protective effects. Briefly, rats were deeply anesthetized with isoflurane. A 30-mm section of the spinal cord was rapidly removed and placed in cold Ringer’s solution (2–4 °C). The dissected dorsal column was exposed to hypoxia with 95% N2 and 5% CO2 and reperfusion with 95% O2 and 5% CO2. The expression of Prdx6 significantly upregulated in white matter after hypoxia compared to the sham group, whereas reperfusion caused a gradual decrease in Prdx6 expression after reperfusion injury. For the first time, our study revealed the novel expression and localized expression of Prdx6 in astrocytes after hypoxia, and possible communication of astrocytes and axons through Prdx6. The gradual increase in Nrf2 expression suggests a negative regulation of Prdx6 through Nrf2 signaling. Furthermore, inhibition of aiPLA2 activity of Prdx6 by MJ33 shows that the regulation of Prdx6 by Nrf2 is mediated through aiPLA2 activity. The present study uncovers a differential distribution of Prdx6 in axons and astrocytes and regulation of Prdx6 in hypoxia-reperfusion injury. The low levels of Prdx6 in reperfusion injury lead to increased inflammation and apoptosis in the white matter; therefore, the results of this study suggest that Prdx6 has a protective role in spinal hypoxia-reperfusion injury.









Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material.
Abbreviations
- aiPLA2:
-
Ca2+-independent phospholipase A2
- ARE:
-
Antioxidant response elements
- cDNA:
-
Complementary DNA
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DC:
-
Dorsal column
- FBS:
-
Fetal bovine serum
- GFAP:
-
Glial fibrillary acidic protein
- H and E:
-
Hematoxylin and eosin
- IACUC:
-
Institutional Animal Care and Use Committee
- KEAP1:
-
Kelch-like ECH-associated protein 1
- MAP 2:
-
Microtubule-associated protein 2
- MJ33:
-
1-Hexadecyl-3-(trifluoroethyl)-sn-glycero-2-phosphomethanol lithium
- NOX2:
-
NADPH oxidase 2
- NF-200:
-
Neurofilament 200
- Nrf2:
-
Nuclear factor erythroid 2–related factor 2
- PBS-T:
-
Phosphate-buffered saline containing 0.1% Tween-20
- Prdx6:
-
Peroxiredoxin-6
- PVDF:
-
Polyvinylidene difluoride
- qRT-PCR:
-
Quantitative real-time PCR
- RIPA:
-
Radioimmunoprecipitation
- ROS:
-
Reactive oxygen species
- RT:
-
Room temperature
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SCI:
-
Spinal cord injury
- TBI:
-
Traumatic brain injury
- TLR4:
-
Toll-like receptor 4
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- UNMC:
-
University of Nebraska Medical Center
- WC:
-
Whole spinal cord
References
Eckert MJ, Martin MJ (2017) Trauma: spinal cord injury. Surg Clin North Am 97(5):1031–1045. https://doi.org/10.1016/j.suc.2017.06.008
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng YL, Cheng PW, Li CY, Li CJ (2018) Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol Biochem 46(4):1650–1667. https://doi.org/10.1159/000489241
Zhu P, Li JX, Fujino M, Zhuang J, Li XK (2013) Development and treatments of inflammatory cells and cytokines in spinal cord ischemia-reperfusion injury. Mediat Inflamm 2013:701970–701977. https://doi.org/10.1155/2013/701970
Fan YD, Zhu ML, Geng D, Zhou K, Du GJ, Wang ZL (2018) The study on pathological mechanism and solution method for spinal cord ischemia reperfusion injury. Eur Rev Med Pharmacol Sci 22(13):4063–4068. https://doi.org/10.26355/eurrev_201807_15394
Zhu H, Santo A, Li Y (2012) The antioxidant enzyme peroxiredoxin and its protective role in neurological disorders. Exp Biol Med (Maywood) 237(2):143–149. https://doi.org/10.1258/ebm.2011.011152
Rhee SG, Chae HZ, Kim K (2005) Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med 38(12):1543–1552. https://doi.org/10.1016/j.freeradbiomed.2005.02.026
Rhee SG, Kang SW, Chang TS, Jeong W, Kim K (2001) Peroxiredoxin, a novel family of peroxidases. IUBMB Life 52(1–2):35–41. https://doi.org/10.1080/15216540252774748
Buonora JE, Mousseau M, Jacobowitz DM, Lazarus RC, Yarnell AM, Olsen CH, Pollard HB, Diaz-Arrastia R et al (2015) Autoimmune profiling reveals peroxiredoxin 6 as a candidate traumatic brain injury biomarker. J Neurotrauma 32(22):1805–1814. https://doi.org/10.1089/neu.2014.3736
Eismann T, Huber N, Shin T, Kuboki S, Galloway E, Wyder M, Edwards MJ, Greis KD et al (2009) Peroxiredoxin-6 protects against mitochondrial dysfunction and liver injury during ischemia-reperfusion in mice. Am J Physiol Gastrointest Liver Physiol 296(2):G266–G274. https://doi.org/10.1152/ajpgi.90583.2008
Elkharaz J, Ugun-Klusek A, Constantin-Teodosiu D, Lawler K, Mayer RJ, Billett E, Lowe J, Bedford L (2013) Implications for oxidative stress and astrocytes following 26S proteasomal depletion in mouse forebrain neurones. Biochim Biophys Acta 1832(12):1930–1938. https://doi.org/10.1016/j.bbadis.2013.07.002
Kim IK, Lee KJ, Rhee S, Seo SB, Pak JH (2013) Protective effects of peroxiredoxin 6 overexpression on amyloid β-induced apoptosis in PC12 cells. Free Radic Res 47(10):836–846. https://doi.org/10.3109/10715762.2013.833330
Manevich Y, Hutchens S, Halushka PV, Tew KD, Townsend DM, Jauch EC, Borg K (2014) Peroxiredoxin VI oxidation in cerebrospinal fluid correlates with traumatic brain injury outcome. Free Radic Biol Med 72:210–221. https://doi.org/10.1016/j.freeradbiomed.2014.04.002
Wagner W, Reuter A, Hüller P, Löwer J, Wessler S (2012) Peroxiredoxin 6 promotes upregulation of the prion protein (PrP) in neuronal cells of prion-infected mice. Cell Commun Signal 10(1):38. https://doi.org/10.1186/1478-811x-10-38
Fisher AB (2019) Antioxidants special issue: peroxiredoxin 6 as a unique member of the peroxiredoxin family. Antioxidants (Basel) 8(4). https://doi.org/10.3390/antiox8040107
Zhou S, Sorokina EM, Harper S, Li H, Ralat L, Dodia C, Speicher DW, Feinstein SI et al (2016) Peroxiredoxin 6 homodimerization and heterodimerization with glutathione S-transferase pi are required for its peroxidase but not phospholipase A2 activity. Free Radic Biol Med 94:145–156. https://doi.org/10.1016/j.freeradbiomed.2016.02.012
Yun HM, Park KR, Kim EC, Hong JT (2015) PRDX6 controls multiple sclerosis by suppressing inflammation and blood brain barrier disruption. Oncotarget 6(25):20875–20884. https://doi.org/10.18632/oncotarget.5205
Daverey A, Agrawal SK (2016) Curcumin alleviates oxidative stress and mitochondrial dysfunction in astrocytes. Neuroscience 333:92–103. https://doi.org/10.1016/j.neuroscience.2016.07.012
Agrawal SK, Fehlings MG (1997) Role of NMDA and non-NMDA ionotropic glutamate receptors in traumatic spinal cord axonal injury. J Neurosci 17(3):1055–1063. https://doi.org/10.1523/jneurosci.17-03-01055.1997
Daverey A, Agrawal SK (2020) Curcumin protects against white matter injury through NF-κB and Nrf2 cross talk. J Neurotrauma 37(10):1255–1265. https://doi.org/10.1089/neu.2019.6749
Wu KC, Cui JY, Klaassen CD (2011) Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol Sci 123(2):590–600. https://doi.org/10.1093/toxsci/kfr183
Kalogeris T, Baines CP, Krenz M, Korthuis RJ (2016) Ischemia/reperfusion. Compr Physiol 7(1):113–170. https://doi.org/10.1002/cphy.c160006
Zhou S, Dodia C, Feinstein SI, Harper S, Forman HJ, Speicher DW, Fisher AB (2018) Oxidation of peroxiredoxin 6 in the presence of GSH increases its phospholipase A2 activity at cytoplasmic pH. Antioxidants (Basel) 8(1). https://doi.org/10.3390/antiox8010004
Yu Q, Huang J, Hu J, Zhu H (2016) Advance in spinal cord ischemia reperfusion injury: blood-spinal cord barrier and remote ischemic preconditioning. Life Sci 154:34–38. https://doi.org/10.1016/j.lfs.2016.03.046
Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS (2001) Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol 24(5):254–264. https://doi.org/10.1097/00002826-200109000-00002
Fisher AB (2017) Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling. Arch Biochem Biophys 617:68–83. https://doi.org/10.1016/j.abb.2016.12.003
Fisher AB, Dodia C, Sorokina EM, Li H, Zhou S, Raabe T, Feinstein SI (2016) A novel lysophosphatidylcholine acyl transferase activity is expressed by peroxiredoxin 6. J Lipid Res 57(4):587–596. https://doi.org/10.1194/jlr.M064758
Power JH, Asad S, Chataway TK, Chegini F, Manavis J, Temlett JA, Jensen PH, Blumbergs PC et al (2008) Peroxiredoxin 6 in human brain: molecular forms, cellular distribution and association with Alzheimer's disease pathology. Acta Neuropathol 115(6):611–622. https://doi.org/10.1007/s00401-008-0373-3
Kuang X, Wang LF, Yu L, Li YJ, Wang YN, He Q, Chen C, Du JR (2014) Ligustilide ameliorates neuroinflammation and brain injury in focal cerebral ischemia/reperfusion rats: involvement of inhibition of TLR4/peroxiredoxin 6 signaling. Free Radic Biol Med 71:165–175. https://doi.org/10.1016/j.freeradbiomed.2014.03.028
Shichita T, Hasegawa E, Kimura A, Morita R, Sakaguchi R, Takada I, Sekiya T, Ooboshi H et al (2012) Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat Med 18(6):911–917. https://doi.org/10.1038/nm.2749
Yeo IJ, Park MH, Son DJ, Kim JY, Nam KT, Hyun BK, Kim SY, Jung MH et al (2019) PRDX6 inhibits neurogenesis through downregulation of WDFY1-mediated TLR4 signal. Mol Neurobiol 56(5):3132–3144. https://doi.org/10.1007/s12035-018-1287-2
Shanshan Y, Beibei J, Li T, Minna G, Shipeng L, Li P, Yong Z (2017) Phospholipase A2 of peroxiredoxin 6 plays a critical role in cerebral ischemia/reperfusion inflammatory injury. Front Cell Neurosci 11:99. https://doi.org/10.3389/fncel.2017.00099
Daverey A, Agrawal SK (2018) Pre and post treatment with curcumin and resveratrol protects astrocytes after oxidative stress. Brain Res 1692:45–55. https://doi.org/10.1016/j.brainres.2018.05.001
Zhang X, Yeung PK, McAlonan GM, Chung SS, Chung SK (2013) Transgenic mice over-expressing endothelial endothelin-1 show cognitive deficit with blood-brain barrier breakdown after transient ischemia with long-term reperfusion. Neurobiol Learn Mem 101:46–54. https://doi.org/10.1016/j.nlm.2013.01.002
Zhang X, Shi LL, Gao X, Jiang D, Zhong ZQ, Zeng X, Rao Y, Hu X et al (2015) Lentivirus-mediated inhibition of tumour necrosis factor-α improves motor function associated with PRDX6 in spinal cord contusion rats. Sci Rep 5:8486. https://doi.org/10.1038/srep08486
Li H, Benipal B, Zhou S, Dodia C, Chatterjee S, Tao JQ, Sorokina EM, Raabe T et al (2015) Critical role of peroxiredoxin 6 in the repair of peroxidized cell membranes following oxidative stress. Free Radic Biol Med 87:356–365. https://doi.org/10.1016/j.freeradbiomed.2015.06.009
Vomund S, Schäfer A, Parnham MJ, Brüne B, von Knethen A (2017) Nrf2, the master regulator of anti-oxidative responses. Int J Mol Sci 18(12). https://doi.org/10.3390/ijms18122772
Bellezza I, Giambanco I, Minelli A, Donato R (2018) Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res 1865(5):721–733. https://doi.org/10.1016/j.bbamcr.2018.02.010
Staurengo-Ferrari L, Badaro-Garcia S, Hohmann MSN, Manchope MF, Zaninelli TH, Casagrande R, Verri WA Jr (2018) Contribution of Nrf2 modulation to the mechanism of action of analgesic and anti-inflammatory drugs in pre-clinical and clinical stages. Front Pharmacol 9:1536. https://doi.org/10.3389/fphar.2018.01536
Lee I, Dodia C, Chatterjee S, Zagorski J, Mesaros C, Blair IA, Feinstein SI, Jain M et al (2013) A novel nontoxic inhibitor of the activation of NADPH oxidase reduces reactive oxygen species production in mouse lung. J Pharmacol Exp Ther 345(2):284–296. https://doi.org/10.1124/jpet.112.201079
Fisher AB (2018) The phospholipase A(2) activity of peroxiredoxin 6. J Lipid Res 59(7):1132–1147. https://doi.org/10.1194/jlr.R082578
Kim SY, Jo HY, Kim MH, Cha YY, Choi SW, Shim JH, Kim TJ, Lee KY (2008) H2O2-dependent hyperoxidation of peroxiredoxin 6 (Prdx6) plays a role in cellular toxicity via up-regulation of iPLA2 activity. J Biol Chem 283(48):33563–33568. https://doi.org/10.1074/jbc.M806578200
Bruno D, Pomara N, Nierenberg J, Ritchie JC, Lutz MW, Zetterberg H, Blennow K (2012) Levels of cerebrospinal fluid neurofilament light protein in healthy elderly vary as a function of TOMM40 variants. Exp Gerontol 47(5):347–352. https://doi.org/10.1016/j.exger.2011.09.008
Acknowledgments
The authors would like to thank the Department of Neurosurgery, University of Nebraska Medical Center for supporting this research.
Author information
Authors and Affiliations
Contributions
AD designed and performed experiments, analyzed and interpreted data, and wrote and revised the manuscript. SA conceived, designed and supervised the project, performed some experiments, analyzed data, and was responsible for data interpretation, and writing and revising the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The animal experiments were carried out in accordance with the UNMC (University of Nebraska Medical Center) Occupational Health and Safety Program for Animal Contact and all the animal procedures used in this study were approved by the IACUC (Institutional Animal Care and Use Committee) of the UNMC.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 1.04 mb)
Rights and permissions
About this article
Cite this article
Daverey, A., Agrawal, S.K. Regulation of Prdx6 by Nrf2 Mediated Through aiPLA2 in White Matter Reperfusion Injury. Mol Neurobiol 58, 1275–1289 (2021). https://doi.org/10.1007/s12035-020-02182-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02182-z