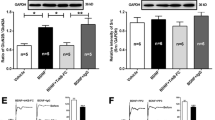
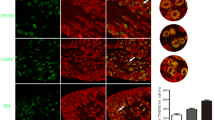
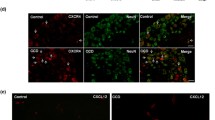
Abstract
At present, chronic post-surgical pain (CPSP) is difficult to prevent and cure clinically because of our lack of understanding of its mechanisms. Surgical injury induces the upregulation of voltage-gated sodium channel Nav1.7 in dorsal root ganglion (DRG) neurons, suggesting that Nav1.7 is involved in the development of CPSP. However, the mechanism leading to persistent dysregulation of Nav1.7 is largely unknown. Given that nerve growth factor (NGF) induces a long-term increase in the neuronal hyperexcitability after injury, we hypothesized that NGF might cause the long-term dysregulation of Nav1.7. In this study, we aimed to investigate whether Nav1.7 regulation by NGF is involved in CPSP and thus contributes to the specific mechanisms involved in the development of CPSP. Using conditional nociceptor-specific Nav1.7 knockout mice, we confirmed the involvement of Nav1.7 in NGF-induced pain and identified its role in the maintenance of pain behavior during long-term observations (up to 14 days). Using western blot analyses and immunostaining, we showed that NGF could trigger the upregulation of Nav1.7 expression and thus support the development of CPSP in rats. Using pharmacological approaches, we showed that the increase of Nav1.7 might be partly regulated by an NGF/TrkA-SGK1-Nedd4-2-mediated pathway. Furthermore, reversing the upregulation of Nav1.7 in DRG could alleviate spinal sensitization. Our results suggest that the maintained upregulation of Nav1.7 triggered by NGF contributes to the development of CPSP. Attenuating the dysregulation of Nav1.7 in peripheral nociceptors may be a strategy to prevent the transition from acute post-surgical pain to CPSP.










Similar content being viewed by others
Abbreviations
- CPSP:
-
chronic post-surgical pain
- Navs:
-
voltage-gated sodium channels
- NGF:
-
nerve growth factor
- DRG:
-
dorsal root ganglion
- PTM:
-
post-translational modification
- Nedd4-2:
-
neuronal precursor cell-expressed developmentally downregulated 4-2
- SGK1:
-
serum and glucocorticoid-inducible kinase
- cKO:
-
conditional knock-out
- SMIR:
-
skin/muscle incision and retraction
- PWT:
-
paw withdrawal threshold
- DMSO:
-
dimethyl sulfoxide
- PLC-γ:
-
phospholipase C-γ
- MAPK:
-
mitogen-activated protein kinase
- PI3K:
-
phosphatidylinositol 3-kinase
- VGLUT2:
-
vesicular glutamate transporter 2
- BDNF:
-
brain-derived neurotrophic factor
- CCL21:
-
chemokine ligand 21
- TrkB:
-
tyrosine kinase receptor B
- CGRP:
-
calcitonin gene-related peptide
- TRPV:
-
transient receptor potential vanilloid family member
- NMDA:
-
N-methyl-D-aspartate receptor
- AMPA:
-
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
References
Wang L, Chang Y, Kennedy SA, Hong PJ, Chow N, Couban RJ, McCabe RE, Bieling PJ et al (2018) Perioperative psychotherapy for persistent post-surgical pain and physical impairment: a meta-analysis of randomised trials. Br J Anaesth 120(6):1304–1314. https://doi.org/10.1016/j.bja.2017.10.026
Chapman CR, Vierck CJ (2017) The transition of acute postoperative pain to chronic pain: an integrative overview of research on mechanisms. J Pain 18(4):359 e351–359 e338. https://doi.org/10.1016/j.jpain.2016.11.004
Poleshuck EL, Katz J, Andrus CH, Hogan LA, Jung BF, Kulick DI, Dworkin RH (2006) Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain 7(9):626–634. https://doi.org/10.1016/j.jpain.2006.02.007
Pluijms WA, Steegers MA, Verhagen AF, Scheffer GJ, Wilder-Smith OH (2006) Chronic post-thoracotomy pain: a retrospective study. Acta Anaesthesiol Scand 50(7):804–808. https://doi.org/10.1111/j.1399-6576.2006.01065.x
Rush AM, Cummins TR, Waxman SG (2007) Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J Physiol 579(Pt 1):1–14. https://doi.org/10.1113/jphysiol.2006.121483
Dib-Hajj SD, Yang Y, Black JA, Waxman SG (2013) The Na(V)1.7 sodium channel: from molecule to man. Nat Rev Neurosci 14(1):49–62. https://doi.org/10.1038/nrn3404
Waxman SG, Merkies ISJ, Gerrits MM, Dib-Hajj SD, Lauria G, Cox JJ, Wood JN, Woods CG et al (2014) Sodium channel genes in pain-related disorders: phenotype-genotype associations and recommendations for clinical use. Lancet Neurol 13(11):1152–1160. https://doi.org/10.1016/S1474-4422(14)70150-4
Black JA, Liu S, Tanaka M, Cummins TR, Waxman SG (2004) Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain 108(3):237–247. https://doi.org/10.1016/j.pain.2003.12.035
Casals-Diaz L, Casas C, Navarro X (2015) Changes of voltage-gated sodium channels in sensory nerve regeneration and neuropathic pain models. Restor Neurol Neurosci 33(3):321–334. https://doi.org/10.3233/RNN-140444
Duan G, Xiang G, Zhang X, Yuan R, Zhan H, Qi D (2013) A single-nucleotide polymorphism in SCN9A may decrease postoperative pain sensitivity in the general population. Anesthesiology 118(2):436–442. https://doi.org/10.1097/ALN.0b013e31827dde74
Sun J, Li N, Duan G, Liu Y, Guo S, Wang C, Zhu C, Zhang X (2018) Increased Nav1.7 expression in the dorsal root ganglion contributes to pain hypersensitivity after plantar incision in rats. Mol Pain 14:1744806918782323. https://doi.org/10.1177/1744806918782323
Li Z, Li Y, Cao J, Han X, Cai W, Zang W, Xu J, Zhang W (2017) Membrane protein Nav1.7 contributes to the persistent post-surgical pain regulated by p-p65 in dorsal root ganglion (DRG) of SMIR rats model. BMC Anesthesiol 17(1):150. https://doi.org/10.1186/s12871-017-0438-8
Basbaum AI, Bautista DM, Scherrer G, Julius D (2009) Cellular and molecular mechanisms of pain. Cell 139(2):267–284. https://doi.org/10.1016/j.cell.2009.09.028
Gaudet AD, Popovich PG, Ramer MS (2011) Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation 8:110. https://doi.org/10.1186/1742-2094-8-110
Leung L, Cahill CM (2010) TNF-alpha and neuropathic pain--a review. J Neuroinflammation 7:27. https://doi.org/10.1186/1742-2094-7-27
Baldelli P, Forni PE, Carbone E (2000) BDNF, NT-3 and NGF induce distinct new Ca2+ channel synthesis in developing hippocampal neurons. Eur J Neurosci 12(11):4017–4032
Wu C, Erickson MA, Xu J, Wild KD, Brennan TJ (2009) Expression profile of nerve growth factor after muscle incision in the rat. Anesthesiology 110(1):140–149. https://doi.org/10.1097/ALN.0b013e318190bc84
Sahbaie P, Shi X, Guo TZ, Qiao Y, Yeomans DC, Kingery WS, Clark JD (2009) Role of substance P signaling in enhanced nociceptive sensitization and local cytokine production after incision. Pain 145(3):341–349. https://doi.org/10.1016/j.pain.2009.06.037
Wu C, Boustany L, Liang H, Brennan TJ (2007) Nerve growth factor expression after plantar incision in the rat. Anesthesiology 107(1):128–135. https://doi.org/10.1097/01.anes.0000267512.08619.bd
Spofford CM, Brennan TJ (2012) Gene expression in skin, muscle, and dorsal root ganglion after plantar incision in the rat. Anesthesiology 117(1):161–172. https://doi.org/10.1097/ALN.0b013e31825a2a2b
Banik RK, Subieta AR, Wu C, Brennan TJ (2005) Increased nerve growth factor after rat plantar incision contributes to guarding behavior and heat hyperalgesia. Pain 117(1–2):68–76. https://doi.org/10.1016/j.pain.2005.05.017
Liu B, Liu Y, Li N, Zhang J, Zhang X (2018) Oxycodone regulates incision-induced activation of neurotrophic factors and receptors in an acute post-surgery pain rat model. J Pain Res 11:2663–2674. https://doi.org/10.2147/JPR.S180396
Toledo-Aral JJ, Moss BL, He ZJ, Koszowski AG, Whisenand T, Levinson SR, Wolf JJ, Silos-Santiago I et al (1997) Identification of PN1, a predominant voltage-dependent sodium channel expressed principally in peripheral neurons. Proc Natl Acad Sci U S A 94(4):1527–1532
Fjell J, Cummins TR, Dib-Hajj SD, Fried K, Black JA, Waxman SG (1999) Differential role of GDNF and NGF in the maintenance of two TTX-resistant sodium channels in adult DRG neurons. Brain Res Mol Brain Res 67(2):267–282
Fjell J, Cummins TR, Davis BM, Albers KM, Fried K, Waxman SG, Black JA (1999) Sodium channel expression in NGF-overexpressing transgenic mice. J Neurosci Res 57(1):39–47. https://doi.org/10.1002/(SICI)1097-4547(19990701)57:1<39::AID-JNR5>3.0.CO;2-M
Brackenbury WJ, Djamgoz MB (2007) Nerve growth factor enhances voltage-gated Na+ channel activity and transwell migration in Mat-LyLu rat prostate cancer cell line. J Cell Physiol 210(3):602–608. https://doi.org/10.1002/jcp.20846
Nassar MA, Stirling LC, Forlani G, Baker MD, Matthews EA, Dickenson AH, Wood JN (2004) Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci U S A 101(34):12706–12711. https://doi.org/10.1073/pnas.0404915101
Atmaramani RR, Black BJ, de la Pena JB, Campbell ZT, Pancrazio JJ (2020) Conserved expression of Nav1.7 and Nav1.8 contribute to the spontaneous and thermally evoked excitability in IL-6 and NGF-sensitized adult dorsal root ganglion neurons in vitro. Bioengineering (Basel) 7(2). https://doi.org/10.3390/bioengineering7020044
Laedermann CJ, Abriel H, Decosterd I (2015) Post-translational modifications of voltage-gated sodium channels in chronic pain syndromes. Front Pharmacol 6:263. https://doi.org/10.3389/fphar.2015.00263
Dib-Hajj SD, Cummins TR, Black JA, Waxman SG (2010) Sodium channels in normal and pathological pain. Annu Rev Neurosci 33:325–347. https://doi.org/10.1146/annurev-neuro-060909-153234
Staub O, Rotin D (2006) Role of ubiquitylation in cellular membrane transport. Physiol Rev 86(2):669–707. https://doi.org/10.1152/physrev.00020.2005
van Bemmelen MX, Rougier JS, Gavillet B, Apotheloz F, Daidie D, Tateyama M, Rivolta I, Thomas MA et al (2004) Cardiac voltage-gated sodium channel Nav1.5 is regulated by Nedd4-2 mediated ubiquitination. Circ Res 95(3):284–291. https://doi.org/10.1161/01.RES.0000136816.05109.89
Fotia AB, Ekberg J, Adams DJ, Cook DI, Poronnik P, Kumar S (2004) Regulation of neuronal voltage-gated sodium channels by the ubiquitin-protein ligases Nedd4 and Nedd4-2. J Biol Chem 279(28):28930–28935. https://doi.org/10.1074/jbc.M402820200
Laedermann CJ, Cachemaille M, Kirschmann G, Pertin M, Gosselin RD, Chang I, Albesa M, Towne C et al (2013) Dysregulation of voltage-gated sodium channels by ubiquitin ligase NEDD4-2 in neuropathic pain. J Clin Invest 123(7):3002–3013. https://doi.org/10.1172/JCI68996
Arevalo JC, Waite J, Rajagopal R, Beyna M, Chen ZY, Lee FS, Chao MV (2006) Cell survival through Trk neurotrophin receptors is differentially regulated by ubiquitination. Neuron 50(4):549–559. https://doi.org/10.1016/j.neuron.2006.03.044
Deumens R, Steyaert A, Forget P, Schubert M, Lavand'homme P, Hermans E, De Kock M (2013) Prevention of chronic postoperative pain: cellular, molecular, and clinical insights for mechanism-based treatment approaches. Prog Neurobiol 104:1–37. https://doi.org/10.1016/j.pneurobio.2013.01.002
Shields SD, Cheng X, Uceyler N, Sommer C, Dib-Hajj SD, Waxman SG (2012) Sodium channel Na(v)1.7 is essential for lowering heat pain threshold after burn injury. J Neurosci 32(32):10819–10832. https://doi.org/10.1523/JNEUROSCI.0304-12.2012
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53(1):55–63
Mills CD, Nguyen T, Tanga FY, Zhong C, Gauvin DM, Mikusa J, Gomez EJ, Salyers AK et al (2013) Characterization of nerve growth factor-induced mechanical and thermal hypersensitivity in rats. Eur J Pain 17(4):469–479. https://doi.org/10.1002/j.1532-2149.2012.00202.x
Flatters SJ (2008) Characterization of a model of persistent postoperative pain evoked by skin/muscle incision and retraction (SMIR). Pain 135(1–2):119–130. https://doi.org/10.1016/j.pain.2007.05.013
Storkson RV, Kjorsvik A, Tjolsen A, Hole K (1996) Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods 65(2):167–172
Ackermann TF, Boini KM, Beier N, Scholz W, Fuchss T, Lang F (2011) EMD638683, a novel SGK inhibitor with antihypertensive potency. Cell Physiol Biochem 28(1):137–146. https://doi.org/10.1159/000331722
Shukla S, Shariat-Madar Z, Walker LA, Tekwani BL (2016) Mechanism for neurotropic action of vorinostat, a pan histone deacetylase inhibitor. Mol Cell Neurosci 77:11–20. https://doi.org/10.1016/j.mcn.2016.09.003
Kudo TA, Kanetaka H, Mochizuki K, Tominami K, Nunome S, Abe G, Kosukegawa H, Abe T et al (2015) Induction of neurite outgrowth in PC12 cells treated with temperature-controlled repeated thermal stimulation. PLoS One 10(4):e0124024. https://doi.org/10.1371/journal.pone.0124024
Liu G, Honisch S, Liu G, Schmidt S, Pantelakos S, Alkahtani S, Toulany M, Lang F et al (2015) Inhibition of SGK1 enhances mAR-induced apoptosis in MCF-7 breast cancer cells. Cancer Biol Ther 16(1):52–59. https://doi.org/10.4161/15384047.2014.986982
Sun R, Liu Y, Hou B, Lei Y, Bo J, Zhang W, Sun Y, Zhang Y et al (2019) Perioperative activation of spinal alpha7 nAChR promotes recovery from preoperative stress-induced prolongation of postsurgical pain. Brain Behav Immun 79:294–308. https://doi.org/10.1016/j.bbi.2019.02.017
Ying YL, Wei XH, Xu XB, She SZ, Zhou LJ, Lv J, Li D, Zheng B et al (2014) Over-expression of P2X7 receptors in spinal glial cells contributes to the development of chronic postsurgical pain induced by skin/muscle incision and retraction (SMIR) in rats. Exp Neurol 261:836–843. https://doi.org/10.1016/j.expneurol.2014.09.007
Peters CM, Eisenach JC (2010) Contribution of the chemokine (C-C motif) ligand 2 (CCL2) to mechanical hypersensitivity after surgical incision in rats. Anesthesiology 112(5):1250–1258. https://doi.org/10.1097/ALN.0b013e3181d3d978
Izumi Y, Sasaki M, Hashimoto S, Sawa T, Amaya F (2015) mTOR signaling controls VGLUT2 expression to maintain pain hypersensitivity after tissue injury. Neuroscience 308:169–179. https://doi.org/10.1016/j.neuroscience.2015.09.013
Li CQ, Xu JM, Liu D, Zhang JY, Dai RP (2008) Brain derived neurotrophic factor (BDNF) contributes to the pain hypersensitivity following surgical incision in the rats. Mol Pain 4:27. https://doi.org/10.1186/1744-8069-4-27
Nijs J, Meeus M, Versijpt J, Moens M, Bos I, Knaepen K, Meeusen R (2015) Brain-derived neurotrophic factor as a driving force behind neuroplasticity in neuropathic and central sensitization pain: a new therapeutic target? Expert Opin Ther Targets 19(4):565–576. https://doi.org/10.1517/14728222.2014.994506
Zhao P, Waxman SG, Hains BC (2007) Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21. J Neurosci 27(33):8893–8902. https://doi.org/10.1523/JNEUROSCI.2209-07.2007
Woolf CJ (2011) Central sensitization: implications for the diagnosis and treatment of pain. Pain 152(3 Suppl):S2–S15. https://doi.org/10.1016/j.pain.2010.09.030
Matzner O, Devor M (1992) Na+ conductance and the threshold for repetitive neuronal firing. Brain Res 597(1):92–98
Hoffmann T, Sharon O, Wittmann J, Carr RW, Vyshnevska A, Col R, Nassar MA, Reeh PW et al (2018) NaV1.7 and pain: Contribution of peripheral nerves. Pain 159(3):496–506. https://doi.org/10.1097/j.pain.0000000000001119
Girard BM, Merrill L, Malley S, Vizzard MA (2013) Increased TRPV4 expression in urinary bladder and lumbosacral dorsal root ganglia in mice with chronic overexpression of NGF in urothelium. J Mol Neurosci 51(2):602–614. https://doi.org/10.1007/s12031-013-0033-5
Shu X, Mendell LM (2001) Acute sensitization by NGF of the response of small-diameter sensory neurons to capsaicin. J Neurophysiol 86(6):2931–2938. https://doi.org/10.1152/jn.2001.86.6.2931
Drew LJ, Rohrer DK, Price MP, Blaver KE, Cockayne DA, Cesare P, Wood JN (2004) Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J Physiol 556(Pt 3):691–710. https://doi.org/10.1113/jphysiol.2003.058693
Canu N, Amadoro G, Triaca V, Latina V, Sposato V, Corsetti V, Severini C, Ciotti MT et al (2017) The intersection of NGF/TrkA signaling and amyloid precursor protein processing in Alzheimer’s disease neuropathology. Int J Mol Sci 18(6). https://doi.org/10.3390/ijms18061319
Isaev NK, Stelmashook EV, Genrikhs EE (2017) Role of nerve growth factor in plasticity of forebrain cholinergic neurons. Biochemistry (Mosc) 82(3):291–300. https://doi.org/10.1134/S0006297917030075
Su R, Su W, Jiao Q (2019) NGF protects neuroblastoma cells against beta-amyloid-induced apoptosis via the Nrf2/HO-1 pathway. FEBS Open Bio 9(12):2063–2071. https://doi.org/10.1002/2211-5463.12742
Dai C, Xiao X, Zhang Y, Xiang B, Hoyer D, Shen J, Velkov T, Tang S (2020) Curcumin attenuates colistin-induced peripheral neurotoxicity in mice. ACS Infect Dis 6(4):715–724. https://doi.org/10.1021/acsinfecdis.9b00341
Zhao G, Ding X, Guo Y, Chen W (2014) Intrathecal lidocaine neurotoxicity: combination with bupivacaine and ropivacaine and effect of nerve growth factor. Life Sci 112(1–2):10–21. https://doi.org/10.1016/j.lfs.2014.07.003
Ferreira RS, Dos Santos NAG, Martins NM, Fernandes LS, Dos Santos AC (2018) Caffeic acid phenethyl ester (CAPE) protects PC12 cells from cisplatin-induced neurotoxicity by activating the NGF-signaling pathway. Neurotox Res 34(1):32–46. https://doi.org/10.1007/s12640-017-9849-z
Cabrera JR, Viejo-Borbolla A, Alcami A, Wandosell F (2016) Secreted herpes simplex virus-2 glycoprotein G alters thermal pain sensitivity by modifying NGF effects on TRPV1. J Neuroinflammation 13(1):210. https://doi.org/10.1186/s12974-016-0677-5
Eskander MA, Ruparel S, Green DP, Chen PB, Por ED, Jeske NA, Gao X, Flores ER et al (2015) Persistent nociception triggered by nerve growth factor (NGF) is mediated by TRPV1 and oxidative mechanisms. J Neurosci 35(22):8593–8603. https://doi.org/10.1523/JNEUROSCI.3993-14.2015
Capsoni S, Brandi R, Arisi I, D'Onofrio M, Cattaneo A (2011) A dual mechanism linking NGF/proNGF imbalance and early inflammation to Alzheimer’s disease neurodegeneration in the AD11 anti-NGF mouse model. CNS Neurol Disord Drug Targets 10(5):635–647. https://doi.org/10.2174/187152711796235032
Kassmann M, Hansel A, Leipold E, Birkenbeil J, Lu SQ, Hoshi T, Heinemann SH (2008) Oxidation of multiple methionine residues impairs rapid sodium channel inactivation. Pflugers Arch 456(6):1085–1095. https://doi.org/10.1007/s00424-008-0477-6
Jeong EM, Liu M, Sturdy M, Gao G, Varghese ST, Sovari AA, Dudley SC Jr (2012) Metabolic stress, reactive oxygen species, and arrhythmia. J Mol Cell Cardiol 52(2):454–463. https://doi.org/10.1016/j.yjmcc.2011.09.018
Schluter F, Leffler A (2016) Oxidation differentially modulates the recombinant voltage-gated Na(+) channel alpha-subunits Nav1.7 and Nav1.8. Brain Res 1648(Pt A):127–135. https://doi.org/10.1016/j.brainres.2016.07.031
Calabrese EJ, Calabrese V, Tsatsakis A, Giordano JJ (2020) Hormesis and Ginkgo biloba (GB): numerous biological effects of GB are mediated via hormesis. Ageing Res Rev:101019. https://doi.org/10.1016/j.arr.2020.101019
Calabrese V, Santoro A, Monti D, Crupi R, Di Paola R, Latteri S, Cuzzocrea S, Zappia M et al (2018) Aging and Parkinson's disease: Inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic Biol Med 115:80–91. https://doi.org/10.1016/j.freeradbiomed.2017.10.379
Calabrese V, Santoro A, Trovato Salinaro A, Modafferi S, Scuto M, Albouchi F, Monti D, Giordano J et al (2018) Hormetic approaches to the treatment of Parkinson's disease: perspectives and possibilities. J Neurosci Res 96(10):1641–1662. https://doi.org/10.1002/jnr.24244
Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP (2010) Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal 13(11):1763–1811. https://doi.org/10.1089/ars.2009.3074
Abriel H, Kamynina E, Horisberger JD, Staub O (2000) Regulation of the cardiac voltage-gated Na+ channel (H1) by the ubiquitin-protein ligase Nedd4. FEBS Lett 466(2–3):377–380
Cachemaille M, Laedermann CJ, Pertin M, Abriel H, Gosselin RD, Decosterd I (2012) Neuronal expression of the ubiquitin ligase Nedd4-2 in rat dorsal root ganglia: modulation in the spared nerve injury model of neuropathic pain. Neuroscience 227:370–380. https://doi.org/10.1016/j.neuroscience.2012.09.044
Boehmer C, Wilhelm V, Palmada M, Wallisch S, Henke G, Brinkmeier H, Cohen P, Pieske B et al (2003) Serum and glucocorticoid inducible kinases in the regulation of the cardiac sodium channel SCN5A. Cardiovasc Res 57(4):1079–1084. https://doi.org/10.1016/s0008-6363(02)00837-4
Snyder PM, Olson DR, Kabra R, Zhou R, Steines JC (2004) cAMP and serum and glucocorticoid-inducible kinase (SGK) regulate the epithelial Na(+) channel through convergent phosphorylation of Nedd4-2. J Biol Chem 279(44):45753–45758. https://doi.org/10.1074/jbc.M407858200
Lang F, Cohen P (2001) Regulation and physiological roles of serum- and glucocorticoid-induced protein kinase isoforms. Sci STKE 2001(108):re17. https://doi.org/10.1126/stke.2001.108.re17
Lang F, Stournaras C, Zacharopoulou N, Voelkl J, Alesutan I (2018) Serum- and glucocorticoid-inducible kinase 1 and the response to cell stress. Cell Stress 3(1):1–8. https://doi.org/10.15698/cst2019.01.170
Marlin MC, Li G (2015) Biogenesis and function of the NGF/TrkA signaling endosome. Int Rev Cell Mol Biol 314:239–257. https://doi.org/10.1016/bs.ircmb.2014.10.002
Frodin M, Antal TL, Dummler BA, Jensen CJ, Deak M, Gammeltoft S, Biondi RM (2002) A phosphoserine/threonine-binding pocket in AGC kinases and PDK1 mediates activation by hydrophobic motif phosphorylation. EMBO J 21(20):5396–5407
Nakagawa T, Yokoe S, Asahi M (2016) Phospholamban degradation is induced by phosphorylation-mediated ubiquitination and inhibited by interaction with cardiac type Sarco(endo)plasmic reticulum Ca(2+)-ATPase. Biochem Biophys Res Commun 472(3):523–530. https://doi.org/10.1016/j.bbrc.2016.03.009
Ni W, Yao S, Zhou Y, Liu Y, Huang P, Zhou A, Liu J, Che L et al (2019) Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m(6)A reader YTHDF3. Mol Cancer 18(1):143. https://doi.org/10.1186/s12943-019-1079-y
Li X, Zhong L, Wang Z, Chen H, Liao D, Zhang R, Zhang H, Kang T (2018) Phosphorylation of IRS4 by CK1gamma2 promotes its degradation by CHIP through the ubiquitin/lysosome pathway. Theranostics 8(13):3643–3653. https://doi.org/10.7150/thno.26021
Peng HY, Chen GD, Hsieh MC, Lai CY, Huang YP, Lin TB (2012) Spinal SGK1/GRASP-1/Rab4 is involved in complete Freund's adjuvant-induced inflammatory pain via regulating dorsal horn GluR1-containing AMPA receptor trafficking in rats. Pain 153(12):2380–2392. https://doi.org/10.1016/j.pain.2012.08.004
Peng HY, Chen GD, Lai CY, Hsieh MC, Lin TB (2013) Spinal serum-inducible and glucocorticoid-inducible kinase 1 mediates neuropathic pain via kalirin and downstream PSD-95-dependent NR2B phosphorylation in rats. J Neurosci 33(12):5227–5240. https://doi.org/10.1523/JNEUROSCI.4452-12.2013
Koyanagi S, Kusunose N, Taniguchi M, Akamine T, Kanado Y, Ozono Y, Masuda T, Kohro Y et al (2016) Glucocorticoid regulation of ATP release from spinal astrocytes underlies diurnal exacerbation of neuropathic mechanical allodynia. Nat Commun 7:13102. https://doi.org/10.1038/ncomms13102
Wen YR, Suter MR, Ji RR, Yeh GC, Wu YS, Wang KC, Kohno T, Sun WZ et al (2009) Activation of p38 mitogen-activated protein kinase in spinal microglia contributes to incision-induced mechanical allodynia. Anesthesiology 110(1):155–165. https://doi.org/10.1097/ALN.0b013e318190bc16
Ito N, Obata H, Saito S (2009) Spinal microglial expression and mechanical hypersensitivity in a postoperative pain model: comparison with a neuropathic pain model. Anesthesiology 111(3):640–648. https://doi.org/10.1097/ALN.0b013e3181b05f42
Di Rosa G, Brunetti G, Scuto M, Trovato Salinaro A, Calabrese EJ, Crea R, Schmitz-Linneweber C, Calabrese V et al (2020) Healthspan enhancement by olive polyphenols in C. elegans wild type and Parkinson's models. Int J Mol Sci 21(11). https://doi.org/10.3390/ijms21113893
Pilipenko V, Narbute K, Amara I, Trovato A, Scuto M, Pupure J, Jansone B, Poikans J et al (2019) GABA-containing compound gammapyrone protects against brain impairments in Alzheimer's disease model male rats and prevents mitochondrial dysfunction in cell culture. J Neurosci Res 97(6):708–726. https://doi.org/10.1002/jnr.24396
Miquel S, Champ C, Day J, Aarts E, Bahr BA, Bakker M, Banati D, Calabrese V et al (2018) Poor cognitive ageing: Vulnerabilities, mechanisms and the impact of nutritional interventions. Ageing Res Rev 42:40–55. https://doi.org/10.1016/j.arr.2017.12.004
Bowtell J, Kelly V (2019) Fruit-derived polyphenol supplementation for athlete recovery and performance. Sports Med 49(Suppl 1):3–23. https://doi.org/10.1007/s40279-018-0998-x
Acknowledgments
We thank Professor Stephen G. Waxman (Yale University School of Medicine, USA) for kindly providing Nav1.7 cKO mice. We would like to thank Editage (www.editage.cn) for the English language editing.
Availability of Data and Material
All data of the study can be acquired from corresponding author through email.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant number: 81271235).
Author information
Authors and Affiliations
Contributions
Study concept and supervision: X.W.Z; study design: X.W.Z and B.W.L; acquisition and analysis: B.W.L, J.Z, Y.S.H, N.B.L, Y.L, M.Z, W.Y.W and H.Z; manuscript preparation: X.W.Z, B.W.L, and A.L.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
All experiments were approved by the ethical committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (IRB ID: TJ-A20180801) and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and followed the guidelines of the International Association for the Study of Pain.
Consent to Participate
Not applicable.
Consent for Publication
All authors consent to the publication of current data.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, BW., Zhang, J., Hong, YS. et al. NGF-Induced Nav1.7 Upregulation Contributes to Chronic Post-surgical Pain by Activating SGK1-Dependent Nedd4-2 Phosphorylation. Mol Neurobiol 58, 964–982 (2021). https://doi.org/10.1007/s12035-020-02156-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02156-1