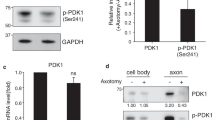
Abstract
Neurons are vulnerable to injury, and failure to activate self-protective systems after injury leads to neuronal death. However, sensory neurons in dorsal root ganglions (DRGs) mostly survive and regenerate their axons. To understand the mechanisms of the neuronal injury response, we analyzed the injury-responsive transcriptome and found that Stc2 is immediately upregulated after axotomy. Stc2 is required for axon regeneration in vivo and in vitro, indicating that Stc2 is a neuronal factor regulating axonal injury response. The application of the secreted stanniocalcin 2 to injured DRG neurons promotes regeneration. Stc2 thus represents a potential secretory protein with a feedback function regulating regeneration. Finally, the in vivo gene delivery of STC2 increases regenerative growth after injury in peripheral nerves in mice. These results suggest that Stc2 is an injury-responsive gene required for axon regeneration and a potential target for developing therapeutic applications.





Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- AU:
-
arbitrary unit
- Ax:
-
axotomy
- AAV:
-
adeno-associated virus
- CM:
-
conditioned medium
- DRB:
-
5,6-dichlorobenzimidazole 1-β-D-ribofuranoside
- DRG:
-
dorsal root ganglion
- HDAC5:
-
histone deacetylase 5
- HDAC5nuc:
-
histone deacetylase 5, nuclear retention mutant form
- HIF-1:
-
hypoxia-inducible factor 1
- Iono:
-
ionomycin
- KD:
-
knockdown
- KO:
-
knockout
- NS:
-
not significant
- PNS:
-
peripheral nervous system
- ShRNA:
-
short hairpin RNA
- SOCE:
-
store-operated calcium entry
- Wk:
-
week
- WT:
-
wild type
References
Gerdts J, Summers DW, Milbrandt J, DiAntonio A (2016) Axon self-destruction: new links among SARM1, MAPKs, and NAD+ metabolism. Neuron 89:449–460. https://doi.org/10.1016/j.neuron.2015.12.023
Wang JT, Medress ZA, Barres BA (2012) Axon degeneration: molecular mechanisms of a self-destruction pathway. J Cell Biol 196:7–18. https://doi.org/10.1083/jcb.201108111
Mahar M, Cavalli V (2018) Intrinsic mechanisms of neuronal axon regeneration. Nat Rev Neurosci 19:323–337. https://doi.org/10.1038/s41583-018-0001-8
Lee J, Shin JE, Lee B, Kim H, Jeon Y, Ahn SH, Chi SW, Cho Y (2020) The stem cell marker Prom1 promotes axon regeneration by down-regulating cholesterol synthesis via Smad signaling. Proc Natl Acad Sci U S A 117:15955–15966. https://doi.org/10.1073/pnas.1920829117
Tedeschi A, Dupraz S, Laskowski CJ, Xue J, Ulas T, Beyer M, Schultze JL, Bradke F (2016) The calcium channel subunit alpha2delta2 suppresses axon regeneration in the adult CNS. Neuron 92:419–434. https://doi.org/10.1016/j.neuron.2016.09.026
Shin JE, Cho Y (2017) Epigenetic regulation of axon regeneration after neural injury. Mol Cells 40. https://doi.org/10.14348/molcells.2017.2311
Shin JE, Ha H, Cho EH, Kim YK, Cho Y (2018) Comparative analysis of the transcriptome of injured nerve segments reveals spatiotemporal responses to neural damage in mice. J Comp Neurol 526:1195–1208. https://doi.org/10.1002/cne.24404
Smith DS, Skene JH (1997) A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J Neurosci 17:646–658
Shin JE, Cho Y, Beirowski B, Milbrandt J, Cavalli V, DiAntonio A (2012) Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron 74:1015–1022. https://doi.org/10.1016/j.neuron.2012.04.028
Shin JE, Ha H, Kim YK, Cho Y, DiAntonio A (2019) DLK regulates a distinctive transcriptional regeneration program after peripheral nerve injury. Neurobiol Dis 127:178–192. https://doi.org/10.1016/j.nbd.2019.02.001
Cho Y, Shin JE, Ewan EE, Oh YM, Pita-Thomas W, Cavalli V (2015) Activating injury-responsive genes with hypoxia enhances axon regeneration through neuronal HIF-1α. Neuron 88:720–734. https://doi.org/10.1016/j.neuron.2015.09.050
Stannius H (1839) Ober Nebenniere bei Knochenfischen. Arch Anat Physiol 6:97–101
Ito D, Walker JR, Thompson CS, Moroz I, Lin W, Veselits ML, Hakim AM, Fienberg AA et al (2004) Characterization of stanniocalcin 2, a novel target of the mammalian unfolded protein response with cytoprotective properties. Mol Cell Biol 24:9456–9469. https://doi.org/10.1128/mcb.24.21.9456-9469.2004
Zeiger W, Ito D, Swetlik C, Oh-hora M, Villereal ML, Thinakaran G (2011) Stanniocalcin 2 is a negative modulator of store-operated calcium entry. Mol Cell Biol 31:3710–3722. https://doi.org/10.1128/mcb.05140-11
Zhang KZ, Lindsberg PJ, Tatlisumak T, Kaste M, Olsen HS, Andersson LC (2000) Stanniocalcin: a molecular guard of neurons during cerebral ischemia. Proc Natl Acad Sci U S A 97:3637–3642. https://doi.org/10.1073/pnas.97.7.3637
Byun JS, Lee JW, Kim SY, Kwon KJ, Sohn JH, Lee K, Oh D, Kim SS et al (2010) Neuroprotective effects of stanniocalcin 2 following kainic acid-induced hippocampal degeneration in ICR mice. Peptides 31:2094–2099. https://doi.org/10.1016/j.peptides.2010.08.002
Cho Y, Sloutsky R, Naegle KM, Cavalli V (2013) Injury-induced HDAC5 nuclear export is essential for axon regeneration. Cell 155:894–908. https://doi.org/10.1016/j.cell.2013.10.004
Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK (2009) Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol 27:59–65. https://doi.org/10.1038/nbt.1515
Cho Y, Di Liberto V, Carlin D et al (2014) Syntaxin13 expression is regulated by mammalian target of rapamycin (mTOR) in injured neurons to promote axon regeneration. J Biol Chem 289:15820–15832. https://doi.org/10.1074/jbc.M113.536607
Song W, Cho Y, Watt D, Cavalli V (2015) Tubulin-tyrosine ligase (TTL)-mediated increase in tyrosinated ??-tubulin in injured axons is required for retrograde injury signaling and axon regeneration. J Biol Chem 290:14765–14775. https://doi.org/10.1074/jbc.M114.622753
Cho Y, Park D, Kim C (2017) Disruption of TACE-filamin interaction can inhibit TACE-mediated ectodomain shedding. Biochem Biophys Res Commun:490. https://doi.org/10.1016/j.bbrc.2017.06.153
Cho Y, Park D, Cavalli V (2015) Filamin a is required in injured axons for HDAC5 activity and axon regeneration. J Biol Chem 290:22759–22770. https://doi.org/10.1074/jbc.M115.638445
Cho Y, Shin JE, Ewan EE, Oh YM, Pita-Thomas W, Cavalli V (2015) Activating injury-responsive genes with hypoxia enhances axon regeneration through neuronal article activating injury-responsive genes with hypoxia enhances axon regeneration through neuronal HIF-1 a. Neuron 88:720–734. https://doi.org/10.1016/j.neuron.2015.09.050
Law AYS, Lai KP, Ip CKM, Wong AST, Wagner GF, Wong CKC (2008) Epigenetic and HIF-1 regulation of stanniocalcin-2 expression in human cancer cells. Exp Cell Res 314:1823–1830. https://doi.org/10.1016/j.yexcr.2008.03.001
Law AYS, Wong CKC (2010) Stanniocalcin-2 is a HIF-1 target gene that promotes cell proliferation in hypoxia. Exp Cell Res 316:466–476. https://doi.org/10.1016/j.yexcr.2009.09.018
Frey E, Valakh V, Karney-Grobe S, Shi Y, Milbrandt J, DiAntonio A (2015) An in vitro assay to study induction of the regenerative state in sensory neurons. Exp Neurol 263:350–363. https://doi.org/10.1016/j.expneurol.2014.10.012
Valakh V, Frey E, Babetto E, Walker LJ, DiAntonio A (2015) Cytoskeletal disruption activates the DLK/JNK pathway, which promotes axonal regeneration and mimics a preconditioning injury. Neurobiol Dis 77:13–25. https://doi.org/10.1016/j.nbd.2015.02.014
Shin JE, Geisler S, DiAntonio A (2014) Dynamic regulation of SCG10 in regenerating axons after injury. Exp Neurol 252:1–11. https://doi.org/10.1016/j.expneurol.2013.11.007
Cho Y, Cavalli V (2012) HDAC5 is a novel injury-regulated tubulin deacetylase controlling axon regeneration. EMBO J 31:3063–3078. https://doi.org/10.1038/emboj.2012.160
Blesch A, Lu P, Tsukada S, Alto LT, Roet K, Coppola G, Geschwind D, Tuszynski MH (2012) Conditioning lesions before or after spinal cord injury recruit broad genetic mechanisms that sustain axonal regeneration: Superiority to camp-mediated effects. Exp Neurol 235:162–173. https://doi.org/10.1016/j.expneurol.2011.12.037
McQuarrie IG, Grafstein B, Dreyfus CF, Gershon MD (1978) Regeneration of adrenergic axons in rat sciatic nerve: effect of a conditioning lesion. Brain Res 141:21–34. https://doi.org/10.1016/0006-8993(78)90614-5
Oudega M, Varon S, Hagg T (1994) Regeneration of adult rat sensory axons into intraspinal nerve grafts: promoting effects of conditioning lesion and graft predegeneration. Exp Neurol 129:194–206. https://doi.org/10.1006/exnr.1994.1161
Sjöberg J, Kanje M (1990) The initial period of peripheral nerve regeneration and the importance of the local environment for the conditioning lesion effect. Brain Res 529:79–84. https://doi.org/10.1016/0006-8993(90)90812-P
Asghari Adib E, Smithson LJ, Collins CA (2018) An axonal stress response pathway: degenerative and regenerative signaling by DLK. Curr Opin Neurobiol 53:110–119. https://doi.org/10.1016/j.conb.2018.07.002
Karney-Grobe S, Russo A, Frey E, Milbrandt J, DiAntonio A (2018) HSP90 is a chaperone for DLK and is required for axon injury signaling. Proc Natl Acad Sci U S A 115:E9899–E9908. https://doi.org/10.1073/pnas.1805351115
Li S, Yang L, Selzer ME, Hu Y (2013) Neuronal endoplasmic reticulum stress in axon injury and neurodegeneration. Ann Neurol 74:768–777. https://doi.org/10.1002/ana.24005
Villanueva MT (2017) Neurodegenerative disease. Nat Rev Drug Discov 16:678–679. https://doi.org/10.1038/nrd.2017.180
Ma W, Quirion R (2017) The ERK/MAPK pathway, as a target for the treatment of neuropathic pain. Expert Opinion on Therapeutic Targets:8222. https://doi.org/10.1517/14728222.9.4.699
Rigaud M, Gemes G, Weyker PD, Cruikshank JM, Kawano T, Wu HE, Hogan QH (2009) Axotomy depletes intracellular calcium stores in primary sensory neurons. Anesthesiology 111:381–392. https://doi.org/10.1097/ALN.0b013e3181ae6212
Ziv NE, Spira ME (1995) Axotomy induces a transient and localized elevation of the free intracellular calcium concentration to the millimolar range. J Neurophysiol 74:2625–2637. https://doi.org/10.1152/jn.1995.74.6.2625
Mandolesi G, MADEDDU F, BOZZI Y et al (2004) Acute physiological response of mammalian central neurons to axotomy: ionic regulation and electrical activity. FASEB J 18:1934–1936. https://doi.org/10.1096/fj.04-1805fje
Wojda U, Salinska E, Kuznicki J (2008) Calcium ions in neuronal degeneration. IUBMB Life 60:575–590. https://doi.org/10.1002/iub.91
Jepsen MR, Kløverpris S, Mikkelsen JH, Pedersen JH, Füchtbauer EM, Laursen LS, Oxvig C (2015) Stanniocalcin-2 inhibits mammalian growth by proteolytic inhibition of the insulin-like growth factor axis. J Biol Chem 290:3430–3439. https://doi.org/10.1074/jbc.M114.611665
Chandran V, Coppola G, Nawabi H, Omura T, Versano R, Huebner EA, Zhang A, Costigan M et al (2016) A systems-level analysis of the peripheral nerve intrinsic axonal growth program. Neuron 89:956–970. https://doi.org/10.1016/j.neuron.2016.01.034
Ma TC, Willis DE (2015) What makes a RAG regeneration associated? Front Mol Neurosci 8:1–13. https://doi.org/10.3389/fnmol.2015.00043
Palmisano I, Di Giovanni S (2018) Advances and limitations of current epigenetic studies investigating mammalian axonal regeneration. Neurotherapeutics 15:529–540. https://doi.org/10.1007/s13311-018-0636-1
Stewart JRR, Thompson MBB, Attaway MBB et al (2006) Of the chorioallantoic placentome and the omphalopleure of the placentotrophic lizard, Pseudemoia entrecasteauxii. J Exp Zool Part a-Comparative Exp Biol 305A:883–889. https://doi.org/10.1002/jez.a
Sazonova O, James KA, McCudden CR et al (2008) Stanniocalcin-1 secretion and receptor regulation in kidney cells. Am J Physiol Ren Physiol 294:788–794. https://doi.org/10.1152/ajprenal.00553.2007
McCudden CR, James KA, Hasilo C, Wagner GF (2002) Characterization of mammalian stanniocalcin receptors: Mitochondrial targeting of ligand and receptor for regulation of cellular metabolism. J Biol Chem 277:45249–45258. https://doi.org/10.1074/jbc.M205954200
McCudden CR, Majewski A, Chakrabarti S, Wagner GF (2004) Co-localization of stanniocalcin-1 ligand and receptor in human breast carcinomas. Mol Cell Endocrinol 213:167–172. https://doi.org/10.1016/j.mce.2003.10.042
Richards TDJ, Fenton AL, Syed R, Wagner GF (2012) Characterization of Stanniocalcin-1 receptors in the rainbow trout. ISRN Endocrinol 2012:1–11. https://doi.org/10.5402/2012/257841
Bano D, Nicotera P (2007) Ca2+ signals and neuronal death in brain ischemia. Stroke 38:674–676. https://doi.org/10.1161/01.STR.0000256294.46009.29
Duchen MR (2012) Mitochondria, calcium-dependent neuronal death and neurodegenerative disease. Pflugers Arch Eur J Physiol 464:111–121. https://doi.org/10.1007/s00424-012-1112-0
Lee J, Byun JS, Lee K, et al (2010) Stanniocalcin 2 Enhances cell proliferation on dentate gyrus of hippocampus in ICR mice. 124–129
Funding
This work was supported by the Health Technology R&D Project (HI17C1459) through the Korea Health Industry Development Institute (KHIDI), funded by the Korean Ministry of Health & Welfare and supported by a Korea University Grant to Y.C., by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIT) 2016R1A5A2007009 to J.E.S.
Author information
Authors and Affiliations
Contributions
J.E.S., V.C., and Y.C. designed the research; J.E.S., Y.J., M.K., E.C., and Y.C. conducted the research; and J.E.S., V.C., and Y.C. wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Ethics Approval
All procedures for animal use were approved by the Korea University Institutional Animal Care & Use Committee (KU-IACUC).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jeon, Y., Shin, J.E., Kwon, M. et al. In Vivo Gene Delivery of STC2 Promotes Axon Regeneration in Sciatic Nerves. Mol Neurobiol 58, 750–760 (2021). https://doi.org/10.1007/s12035-020-02155-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02155-2