Abstract
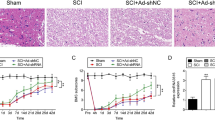
Accidents are the cause of some 50 deaths per 100,000 population each year; some 3% of these are from traumatic spinal cord injury (SCI), a damage that causes temporary or permanent motor deficits, often leading to permanent neurological alterations. The activation of poly(ADP-ribose) polymerase (PARP) as DNA damage response, together with autophagy and apoptosis processes contributes to the secondary injury processes seen after SCI. Thus, in the present study, a mouse compression model of SCI was used to determine whether the treatment with ABT888, as PARP-1/2 inhibitor, could restore the neuronal damage induced by SCI. Mice were orally administered with ABT888 (at a dose of 25 mg/kg) 1 h and 6 h after SCI induction. Histological analysis, myeloperoxidase (MPO) activity, and Basso Mouse scale (BMS) were performed. The expression of autophagy-related proteins and apoptosis-inducing factors was quantified in the cytosolic fraction from spinal cord tissue collected after 24 h after SCI. TUNEL assay was performed in SCI-tissues 24 h after damage. ABT888 treatment significantly reduced histological damage and neutrophilic infiltration, improving motor skills. PARP-1/2 inhibition by ABT888 slowed cell death, decreasing autophagy-activation proteins. These results showed that ABT888, inhibiting PARP-1/2 activity, through a reduction in the apoptosis-autophagy machinery, plays a protective role after SCI, suggesting a new insight into the potential application of ABT888 as novel candidate in SCI therapies.








Similar content being viewed by others
Data Availability
(Not applicable)
References
Sezer N, Akkus S, Ugurlu FG (2015) Chronic complications of spinal cord injury. World J Orthop 6(1):24–33. https://doi.org/10.5312/wjo.v6.i1.24
Liu L, Zhou J, Wang Y, Qi T, Wang Z, Chen L, Suo N (2020) Imatinib inhibits oxidative stress response in spinal cord injury rats by activating Nrf2/HO-1 signaling pathway. Exp Ther Med 19(1):597–602. https://doi.org/10.3892/etm.2019.8270
Islam BU, Habib S, Ahmad P, Allarakha S, Moinuddin AA (2015) Pathophysiological role of peroxynitrite induced DNA damage in human diseases: a special focus on poly(ADP-ribose) polymerase (PARP). Indian J Clin Biochem 30(4):368–385. https://doi.org/10.1007/s12291-014-0475-8
Joly LM, Benjelloun N, Plotkine M, Charriaut-Marlangue C (2003) Distribution of poly(ADP-ribosyl)ation and cell death after cerebral ischemia in the neonatal rat. Pediatr Res 53(5):776–782. https://doi.org/10.1203/01.PDR.0000059751.00465.F6
de Murcia JM, Niedergang C, Trucco C, Ricoul M, Dutrillaux B, Mark M, Oliver FJ, Masson M et al (1997) Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc Natl Acad Sci U S A 94(14):7303–7307. https://doi.org/10.1073/pnas.94.14.7303
Cipriani G, Rapizzi E, Vannacci A, Rizzuto R, Moroni F, Chiarugi A (2005) Nuclear poly(ADP-ribose) polymerase-1 rapidly triggers mitochondrial dysfunction. J Biol Chem 280(17):17227–17234. https://doi.org/10.1074/jbc.M414526200
Dotiwala F, Eapen VV, Harrison JC, Arbel-Eden A, Ranade V, Yoshida S, Haber JE (2013) DNA damage checkpoint triggers autophagy to regulate the initiation of anaphase. Proc Natl Acad Sci U S A 110(1):E41–E49. https://doi.org/10.1073/pnas.1218065109
Surova O, Zhivotovsky B (2013) Various modes of cell death induced by DNA damage. Oncogene 32(33):3789–3797. https://doi.org/10.1038/onc.2012.556
Glick D, Barth S, Macleod KF (2010) Autophagy: cellular and molecular mechanisms. J Pathol 221(1):3–12. https://doi.org/10.1002/path.2697
Yang Z, Klionsky DJ (2010) Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol 22(2):124–131. https://doi.org/10.1016/j.ceb.2009.11.014
Levine B, Klionsky DJ (2004) Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6(4):463–477. https://doi.org/10.1016/s1534-5807(04)00099-1
Cherra SJ 3rd, Chu CT (2008) Autophagy in neuroprotection and neurodegeneration: a question of balance. Future Neurol 3(3):309–323. https://doi.org/10.2217/14796708.3.3.309
Tsujimoto Y, Shimizu S (2005) Another way to die: autophagic programmed cell death. Cell Death Differ 12(Suppl 2):1528–1534. https://doi.org/10.1038/sj.cdd.4401777
Huang ML, Chiang S, Kalinowski DS, Bae DH, Sahni S, Richardson DR (2019) The role of the antioxidant response in mitochondrial dysfunction in degenerative diseases: cross-talk between antioxidant defense, autophagy, and apoptosis. Oxidative Med Cell Longev 2019:6392763–6392726. https://doi.org/10.1155/2019/6392763
Tuli R, Shiao SL, Nissen N, Tighiouart M, Kim S, Osipov A, Bryant M, Ristow L et al (2019) A phase 1 study of veliparib, a PARP-1/2 inhibitor, with gemcitabine and radiotherapy in locally advanced pancreatic cancer. EBioMedicine 40:375–381. https://doi.org/10.1016/j.ebiom.2018.12.060
Virag L, Szabo C (2002) The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev 54(3):375–429. https://doi.org/10.1124/pr.54.3.375
Southan GJ, Szabo C (2003) Poly(ADP-ribose) polymerase inhibitors. Curr Med Chem 10(4):321–340. https://doi.org/10.2174/0929867033368376
Paterniti I, Esposito E, Cuzzocrea S (2018) An in vivo compression model of spinal cord injury. Methods Mol Biol 1727:379–384. https://doi.org/10.1007/978-1-4939-7571-6_29
Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG (2006) Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 23(5):635–659. https://doi.org/10.1089/neu.2006.23.635
Siracusa R, Paterniti I, Bruschetta G, Cordaro M, Impellizzeri D, Crupi R, Cuzzocrea S, Esposito E (2016) The association of palmitoylethanolamide with luteolin decreases autophagy in spinal cord injury. Mol Neurobiol 53(6):3783–3792. https://doi.org/10.1007/s12035-015-9328-6
Casili G, Cordaro M, Impellizzeri D, Bruschetta G, Paterniti I, Cuzzocrea S, Esposito E (2016) Dimethyl fumarate reduces inflammatory responses in experimental colitis. J Crohns Colitis 10(4):472–483. https://doi.org/10.1093/ecco-jcc/jjv231
Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD, Yezierski RP (1998) Traumatic spinal cord injury induces nuclear factor-kappaB activation. J Neurosci 18(9):3251–3260
Leinhase I, Holers VM, Thurman JM, Harhausen D, Schmidt OI, Pietzcker M, Taha ME, Rittirsch D et al (2006) Reduced neuronal cell death after experimental brain injury in mice lacking a functional alternative pathway of complement activation. BMC Neurosci 7:55. https://doi.org/10.1186/1471-2202-7-55
Oliveira KM, Binda NS, Lavor MSL, Silva CMO, Rosado IR, Gabellini ELA, Da Silva JF, Oliveira CM et al (2018) Conotoxin MVIIA improves cell viability and antioxidant system after spinal cord injury in rats. PLoS One 13(10):e0204948. https://doi.org/10.1371/journal.pone.0204948
Taoka Y, Okajima K, Uchiba M, Murakami K, Kushimoto S, Johno M, Naruo M, Okabe H et al (1997) Role of neutrophils in spinal cord injury in the rat. Neuroscience 79(4):1177–1182. https://doi.org/10.1016/s0306-4522(97)00011-0
Sakoh-Nakatogawa M, Matoba K, Asai E, Kirisako H, Ishii J, Noda NN, Inagaki F, Nakatogawa H et al (2013) Atg12-Atg5 conjugate enhances E2 activity of Atg3 by rearranging its catalytic site. Nat Struct Mol Biol 20(4):433–439. https://doi.org/10.1038/nsmb.2527
Ribas VT, Lingor P (2015) Autophagy in degenerating axons following spinal cord injury: evidence for autophagosome biogenesis in retraction bulbs. Neural Regen Res 10(2):198–200. https://doi.org/10.4103/1673-5374.152367
Barth S, Glick D, Macleod KF (2010) Autophagy: assays and artifacts. J Pathol 221(2):117–124. https://doi.org/10.1002/path.2694
Komatsu M, Ichimura Y (2010) Physiological significance of selective degradation of p62 by autophagy. FEBS Lett 584(7):1374–1378. https://doi.org/10.1016/j.febslet.2010.02.017
Jia Z, Zhu H, Li J, Wang X, Misra H, Li Y (2012) Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord 50(4):264–274. https://doi.org/10.1038/sc.2011.111
Filomeni G, De Zio D, Cecconi F (2015) Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ 22(3):377–388. https://doi.org/10.1038/cdd.2014.150
Bains M, Hall ED (2012) Antioxidant therapies in traumatic brain and spinal cord injury. Biochim Biophys Acta 1822(5):675–684. https://doi.org/10.1016/j.bbadis.2011.10.017
Cao B, Li J, Zhou X, Juan J, Han K, Zhang Z, Kong Y, Wang J et al (2014) Clioquinol induces pro-death autophagy in leukemia and myeloma cells by disrupting the mTOR signaling pathway. Sci Rep 4:5749. https://doi.org/10.1038/srep05749
Tapodi A, Bognar Z, Szabo C, Gallyas F, Sumegi B, Hocsak E (2019) PARP inhibition induces Akt-mediated cytoprotective effects through the formation of a mitochondria-targeted phospho-ATM-NEMO-Akt-mTOR signalosome. Biochem Pharmacol 162:98–108. https://doi.org/10.1016/j.bcp.2018.10.005
Huang Z, Xu W, Wu J, Chen S, Chen X (2018) The role of PI3-K/Akt signal pathway in the antagonist effect of CEPO on CHF rats. Exp Ther Med 16(6):5161–5165. https://doi.org/10.3892/etm.2018.6822
Wiman KG (2013) p53 talks to PARP: The increasing complexity of p53-induced cell death. Cell Death Differ 20(11):1438–1439. https://doi.org/10.1038/cdd.2013.111
Fridman JS, Lowe SW (2003) Control of apoptosis by p53. Oncogene 22(56):9030–9040. https://doi.org/10.1038/sj.onc.1207116
Chen HC, Fong TH, Lee AW, Chiu WT (2012) Autophagy is activated in injured neurons and inhibited by methylprednisolone after experimental spinal cord injury. Spine (Phila Pa 1976) 37(6):470–475. https://doi.org/10.1097/BRS.0b013e318221e859
Green DR, Llambi F (2015) Cell death signaling. Cold Spring Harb Perspect Biol 7(12). https://doi.org/10.1101/cshperspect.a006080
Kroemer G, Marino G, Levine B (2010) Autophagy and the integrated stress response. Mol Cell 40(2):280–293. https://doi.org/10.1016/j.molcel.2010.09.023
Wang X, Sekine Y, Byrne AB, Cafferty WB, Hammarlund M, Strittmatter SM (2016) Inhibition of poly-ADP-Ribosylation fails to increase axonal regeneration or improve functional recovery after adult mammalian CNS injury. eNeuro 3(6):ENEURO.0270–ENEU16.2016. https://doi.org/10.1523/ENEURO.0270-16.2016
Palma JP, Rodriguez LE, Bontcheva-Diaz VD, Bouska JJ, Bukofzer G, Colon-Lopez M, Guan R, Jarvis K et al (2008) The PARP inhibitor, ABT-888 potentiates temozolomide: Correlation with drug levels and reduction in PARP activity in vivo. Anticancer Res 28(5A):2625–2635
Munoz-Gamez JA, Rodriguez-Vargas JM, Quiles-Perez R, Aguilar-Quesada R, Martin-Oliva D, de Murcia G, Menissier de Murcia J, Almendros A et al (2009) PARP-1 is involved in autophagy induced by DNA damage. Autophagy 5(1):61–74. https://doi.org/10.4161/auto.5.1.7272
Paterniti I, Impellizzeri D, Di Paola R, Esposito E, Gladman S, Yip P, Priestley JV, Michael-Titus AT et al (2014) Docosahexaenoic acid attenuates the early inflammatory response following spinal cord injury in mice: in-vivo and in-vitro studies. J Neuroinflammation 11:6. https://doi.org/10.1186/1742-2094-11-6
Casili G, Impellizzeri D, Cordaro M, Esposito E, Cuzzocrea S (2016) B-cell depletion with CD20 antibodies as new approach in the treatment of inflammatory and immunological events associated with spinal cord injury. Neurotherapeutics 13(4):880–894. https://doi.org/10.1007/s13311-016-0446-2
McGurk L, Mojsilovic-Petrovic J, Van Deerlin VM, Shorter J, Kalb RG, Lee VM, Trojanowski JQ, Lee EB et al (2018) Nuclear poly(ADP-ribose) activity is a therapeutic target in amyotrophic lateral sclerosis. Acta Neuropathol Commun 6(1):84. https://doi.org/10.1186/s40478-018-0586-1
Khalatbary AR, Ahmadvand H (2011) Effect of oleuropein on tissue myeloperoxidase activity in experimental spinal cord trauma. Iran Biomed J 15(4):164–167. https://doi.org/10.6091/ibj.1026.2012
Jagtap P, Szabo C (2005) Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov 4(5):421–440. https://doi.org/10.1038/nrd1718
Zhou KL, Zhang XL, Wu K, Wang YL, Xu HZ (2015) Progress on the role of autophagy in spinal cord injury. Zhongguo Gu Shang 28(8):695–698
Keller CW, Lunemann JD (2017) Autophagy and autophagy-related proteins in CNS autoimmunity. Front Immunol 8:165. https://doi.org/10.3389/fimmu.2017.00165
Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI et al (2010) Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev 90(4):1383–1435. https://doi.org/10.1152/physrev.00030.2009
Rodriguez-Vargas JM, Rodriguez MI, Majuelos-Melguizo J, Garcia-Diaz A, Gonzalez-Flores A, Lopez-Rivas A, Virag L, Illuzzi G et al (2016) Autophagy requires poly(adp-ribosyl)ation-dependent AMPK nuclear export. Cell Death Differ 23(12):2007–2018. https://doi.org/10.1038/cdd.2016.80
Tanabe F, Yone K, Kawabata N, Sakakima H, Matsuda F, Ishidou Y, Maeda S, Abematsu M et al (2011) Accumulation of p62 in degenerated spinal cord under chronic mechanical compression: functional analysis of p62 and autophagy in hypoxic neuronal cells. Autophagy 7(12):1462–1471. https://doi.org/10.4161/auto.7.12.17892
Talloczy Z, Jiang W, Virgin HW, Leib DA, Scheuner D, Kaufman RJ, Eskelinen EL, Levine B (2002) Regulation of starvation- and virus-induced autophagy by the eIF2alpha kinase signaling pathway. Proc Natl Acad Sci U S A 99(1):190–195. https://doi.org/10.1073/pnas.012485299
Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, Dunn RS, Vorhees CV et al (2006) Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol 169(2):566–583. https://doi.org/10.2353/ajpath.2006.051066
Tanida I, Ueno T, Kominami E (2004) LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol 36(12):2503–2518. https://doi.org/10.1016/j.biocel.2004.05.009
Liu WJ, Ye L, Huang WF, Guo LJ, Xu ZG, Wu HL, Yang C, Liu HF (2016) p62 links the autophagy pathway and the ubiqutin-proteasome system upon ubiquitinated protein degradation. Cell Mol Biol Lett 21:29. https://doi.org/10.1186/s11658-016-0031-z
Korolchuk VI, Mansilla A, Menzies FM, Rubinsztein DC (2009) Autophagy inhibition compromises degradation of ubiquitin-proteasome pathway substrates. Mol Cell 33(4):517–527. https://doi.org/10.1016/j.molcel.2009.01.021
Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171(4):603–614. https://doi.org/10.1083/jcb.200507002
Sahani MH, Itakura E, Mizushima N (2014) Expression of the autophagy substrate SQSTM1/p62 is restored during prolonged starvation depending on transcriptional upregulation and autophagy-derived amino acids. Autophagy 10(3):431–441. https://doi.org/10.4161/auto.27344
Rodriguez-Vargas JM, Ruiz-Magana MJ, Ruiz-Ruiz C, Majuelos-Melguizo J, Peralta-Leal A, Rodriguez MI, Munoz-Gamez JA, de Almodovar MR et al (2012) ROS-induced DNA damage and PARP-1 are required for optimal induction of starvation-induced autophagy. Cell Res 22(7):1181–1198. https://doi.org/10.1038/cr.2012.70
Barrera G (2012) Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol 2012:137289–137221. https://doi.org/10.5402/2012/137289
Tapodi A, Debreceni B, Hanto K, Bognar Z, Wittmann I, Gallyas F Jr, Varbiro G, Sumegi B (2005) Pivotal role of Akt activation in mitochondrial protection and cell survival by poly(ADP-ribose)polymerase-1 inhibition in oxidative stress. J Biol Chem 280(42):35767–35775. https://doi.org/10.1074/jbc.M507075200
Szanto A, Hellebrand EE, Bognar Z, Tucsek Z, Szabo A, Gallyas F Jr, Sumegi B, Varbiro G (2009) PARP-1 inhibition-induced activation of PI-3-kinase-Akt pathway promotes resistance to taxol. Biochem Pharmacol 77(8):1348–1357. https://doi.org/10.1016/j.bcp.2009.01.008
Lu H, Zhang LH, Yang L, Tang PF (2018) The PI3K/Akt/FOXO3a pathway regulates regeneration following spinal cord injury in adult rats through TNF-alpha and p27kip1 expression. Int J Mol Med 41(5):2832–2838. https://doi.org/10.3892/ijmm.2018.3459
Dong M, Yang G, Liu H, Liu X, Lin S, Sun D, Wang Y (2014) Aged black garlic extract inhibits HT29 colon cancer cell growth via the PI3K/Akt signaling pathway. Biomed Rep 2(2):250–254. https://doi.org/10.3892/br.2014.226
Singh B, Reddy PG, Goberdhan A, Walsh C, Dao S, Ngai I, Chou TC, P OC et al (2002) p53 regulates cell survival by inhibiting PIK3CA in squamous cell carcinomas. Genes Dev 16(8):984–993. https://doi.org/10.1101/gad.973602
Burkhardt JK, Bozinov O, Nurnberg J, Shin B, Woernle CM, Ulrich NH, Bertalanffy H (2012) Neurosurgical considerations on highly eloquent brainstem cavernomas during pregnancy. Clin Neurol Neurosurg 114(8):1172–1176. https://doi.org/10.1016/j.clineuro.2012.02.040
Kotipatruni RR, Dasari VR, Veeravalli KK, Dinh DH, Fassett D, Rao JS (2011) p53- and Bax-mediated apoptosis in injured rat spinal cord. Neurochem Res 36(11):2063–2074. https://doi.org/10.1007/s11064-011-0530-2
Oliver FJ, Menissier-de Murcia J, de Murcia G (1999) Poly(ADP-ribose) polymerase in the cellular response to DNA damage, apoptosis, and disease. Am J Hum Genet 64(5):1282–1288. https://doi.org/10.1086/302389
Schuler M, Bossy-Wetzel E, Goldstein JC, Fitzgerald P, Green DR (2000) p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J Biol Chem 275(10):7337–7342. https://doi.org/10.1074/jbc.275.10.7337
Kobayashi D, Tokino T, Watanabe N (2001) Contribution of caspase-3 differs by p53 status in apoptosis induced by X-irradiation. Jpn J Cancer Res 92(4):475–481. https://doi.org/10.1111/j.1349-7006.2001.tb01118.x
Zhang F, Lau SS, Monks TJ (2012) A dual role for poly(ADP-ribose) polymerase-1 during caspase-dependent apoptosis. Toxicol Sci 128(1):103–114. https://doi.org/10.1093/toxsci/kfs142
Tait SW, Green DR (2008) Caspase-independent cell death: leaving the set without the final cut. Oncogene 27(50):6452–6461. https://doi.org/10.1038/onc.2008.311
Chen T, Wang W, Li JR, Xu HZ, Peng YC, Fan LF, Yan F, Gu C et al (2016) PARP inhibition attenuates early brain injury through NF-kappaB/MMP-9 pathway in a rat model of subarachnoid hemorrhage. Brain Res 1644:32–38. https://doi.org/10.1016/j.brainres.2016.05.005
Arnoult D, Gaume B, Karbowski M, Sharpe JC, Cecconi F, Youle RJ (2003) Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J 22(17):4385–4399. https://doi.org/10.1093/emboj/cdg423
Kyrylkova K, Kyryachenko S, Leid M, Kioussi C (2012) Detection of apoptosis by TUNEL assay. Methods Mol Biol 887:41–47. https://doi.org/10.1007/978-1-61779-860-3_5
Kane DJ, Sarafian TA, Anton R, Hahn H, Gralla EB, Valentine JS, Ord T, Bredesen DE (1993) Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science 262(5137):1274–1277. https://doi.org/10.1126/science.8235659
Wang Y, Sun ZY, Zhang KM, Xu GQ, Li G (2011) Bcl-2 in suppressing neuronal apoptosis after spinal cord injury. World J Emerg Med 2(1):38–44
Author information
Authors and Affiliations
Contributions
GC: conceptualization, data curation, formal analysis, and writing-original draft; MC: conceptualization, data curation, methodology, and resources; ML: formal analysis, investigation; AF: investigation, methodology; SS: methodology; AA: software; EE: conceptualization, resources, funding acquisition, and supervision; and IP: conceptualization, validation, resources, visualization, and project administration.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Code Availability
(Not applicable)
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Casili, G., Campolo, M., Lanza, M. et al. Role of ABT888, a Novel Poly(ADP-Ribose) Polymerase (PARP) Inhibitor in Countering Autophagy and Apoptotic Processes Associated to Spinal Cord Injury. Mol Neurobiol 57, 4394–4407 (2020). https://doi.org/10.1007/s12035-020-02033-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02033-x