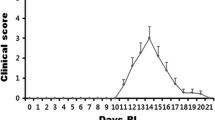
Abstract
Cathepsins are a family of lysosomal/endosomal proteolytic enzymes that include serine, aspartate, and cysteine proteases. The role of cathepsin in neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease, remains elusive. We evaluated the expression level and localization of different cathepsins in the olfactory bulbs of mice with experimental autoimmune encephalomyelitis (EAE), a model of human multiple sclerosis. Quantitative real-time PCR results and Western blotting analyses revealed that serine, aspartate, and cysteine cathepsins are expressed at significantly higher levels in the olfactory bulbs of mice with EAE in the paralytic stage compared with those of control mice. Immunohistochemical analyses indicated that cathepsin A, D, and S were expressed in the glomerulus layer, external plexiform layer, and mitral cell layer. Furthermore, cathepsins were detected in astrocytes, microglia, inflammatory cells, and vascular cells in the olfactory bulb of EAE mice at the paralytic stage. Collectively, these results suggest that the upregulation of cathepsins in the olfactory bulb of mice with EAE is associated with transient olfactory dysfunction in autoimmune encephalomyelitis.





Similar content being viewed by others
Abbreviations
- CNS:
-
Central nervous system
- EAE:
-
Experimental autoimmune encephalomyelitis
- GFAP:
-
Glial fibrillary acidic protein
- Iba1:
-
Ionized calcium binding adaptor molecule 1
- IB4:
-
Isolectin-B4
- IL:
-
Interleukin
- MOG:
-
Myelin oligodendrocyte glycoprotein
- MS:
-
Multiple sclerosis
- PBS:
-
Phosphate-buffered saline
- PI:
-
Post-immunization
References
Doorn KJ, Goudriaan A, Blits-Huizinga C, Bol JG, Rozemuller AJ, Hoogland PV, Lucassen PJ, Drukarch B et al (2014) Increased amoeboid microglial density in the olfactory bulb of Parkinson’s and Alzheimer’s patients. Brain Pathol 24(2):152–165. https://doi.org/10.1111/bpa.12088
Kohl Z, Schlachetzki JC, Feldewerth J, Hornauer P, Munch M, Adame A, Riemenschneider MJ, Winkler J et al (2017) Distinct pattern of microgliosis in the olfactory bulb of neurodegenerative proteinopathies. Neural Plast 2017:3851262. https://doi.org/10.1155/2017/3851262
Uecker FC, Olze H, Kunte H, Gerz C, Goktas O, Harms L, Schmidt FA (2017) Longitudinal testing of olfactory and gustatory function in patients with multiple sclerosis. PLoS One 12(1):e0170492. https://doi.org/10.1371/journal.pone.0170492
Shin T, Kim J, Ahn M, Moon C (2018) Olfactory dysfunction in CNS neuroimmunological disorders: a review. Mol Neurobiol. https://doi.org/10.1007/s12035-018-1341-0
Bombini MF, Peres FA, Lapa AT, Sinicato NA, Quental BR, Pincelli ASM, Amaral TN, Gomes CC et al (2018) Olfactory function in systemic lupus erythematosus and systemic sclerosis. A longitudinal study and review of the literature. Autoimmun Rev 17(4):405–412. https://doi.org/10.1016/j.autrev.2018.02.002
Doty RL, Li C, Mannon LJ, Yousem DM (1998) Olfactory dysfunction in multiple sclerosis. Relation to plaque load in inferior frontal and temporal lobes. Ann N Y Acad Sci 855:781–786
Seo Y, Kim HS, Kang I, Choi SW, Shin TH, Shin JH, Lee BC, Lee JY et al (2016) Cathepsin S contributes to microglia-mediated olfactory dysfunction through the regulation of Cx3cl1-Cx3cr1 axis in a Niemann-Pick disease type C1 model. Glia 64(12):2291–2305. https://doi.org/10.1002/glia.23077
Hovakimyan M, Meyer A, Lukas J, Luo J, Gudziol V, Hummel T, Rolfs A, Wree A et al (2013) Olfactory deficits in Niemann-Pick type C1 (NPC1) disease. PLoS One 8(12):e82216. https://doi.org/10.1371/journal.pone.0082216
Kim J, Choi Y, Ahn M, Jung K, Shin T (2018) Olfactory dysfunction in autoimmune central nervous system neuroinflammation. Mol Neurobiol 55(11):8499–8508. https://doi.org/10.1007/s12035-018-1001-4
Shin T, Kojima T, Tanuma N, Ishihara Y, Matsumoto Y (1995) The subarachnoid space as a site for precursor T cell proliferation and effector T cell selection in experimental autoimmune encephalomyelitis. J Neuroimmunol 56(2):171–178
Kim J, Ahn M, Choi Y, Ekanayake P, Park CM, Moon C, Jung K, Tanaka A et al (2019) Gene expression profile of olfactory transduction signaling in an animal model of human multiple sclerosis. Exp Neurobiol 28(1):74–84
Cocchiaro P, De Pasquale V, Della Morte R, Tafuri S, Avallone L, Pizard A, Moles A, Pavone LM (2017) The multifaceted role of the lysosomal protease cathepsins in kidney disease. Front Cell Dev Biol 5:114. https://doi.org/10.3389/fcell.2017.00114
Stoka V, Turk V, Turk B (2016) Lysosomal cathepsins and their regulation in aging and neurodegeneration. Ageing Res Rev 32:22–37. https://doi.org/10.1016/j.arr.2016.04.010
Schechter I, Ziv E (2011) Cathepsins S, B and L with aminopeptidases display beta-secretase activity associated with the pathogenesis of Alzheimer’s disease. Biol Chem 392(6):555–569. https://doi.org/10.1515/BC.2011.054
Bordi M, Berg MJ, Mohan PS, Peterhoff CM, Alldred MJ, Che S, Ginsberg SD, Nixon RA (2016) Autophagy flux in CA1 neurons of Alzheimer hippocampus: increased induction overburdens failing lysosomes to propel neuritic dystrophy. Autophagy 12(12):2467–2483. https://doi.org/10.1080/15548627.2016.1239003
Li L, Wang X, Fei X, Xia L, Qin Z, Liang Z (2011) Parkinson’s disease involves autophagy and abnormal distribution of cathepsin L. Neurosci Lett 489(1):62–67. https://doi.org/10.1016/j.neulet.2010.11.068
Benes P, Vetvicka V, Fusek M (2008) Cathepsin D--many functions of one aspartic protease. Crit Rev Oncol Hematol 68(1):12–28. https://doi.org/10.1016/j.critrevonc.2008.02.008
Haves-Zburof D, Paperna T, Gour-Lavie A, Mandel I, Glass-Marmor L, Miller A (2011) Cathepsins and their endogenous inhibitors cystatins: expression and modulation in multiple sclerosis. J Cell Mol Med 15(11):2421–2429. https://doi.org/10.1111/j.1582-4934.2010.01229.x
Kim H, Ahn M, Moon C, Matsumoto Y, Sung Koh C, Shin T (2006) Immunohistochemical study of flotillin-1 in the spinal cord of Lewis rats with experimental autoimmune encephalomyelitis. Brain Res 1114(1):204–211. https://doi.org/10.1016/j.brainres.2006.07.054
Ahn M, Kim J, Park C, Jung K, Moon C, Shin T (2015) Immunohistochemical study of Kruppel-like factor 4 in the spinal cords of rats with experimental autoimmune encephalomyelitis. Acta Histochem 117(6):521–527. https://doi.org/10.1016/j.acthis.2015.03.012
Choi Y, Kim J, Ahn M, Shin T (2020) Upregulation of periostin in MOG-induced experimental autoimmune encephalomyelitis in mice. Neurosci Lett 715:134558. https://doi.org/10.1016/j.neulet.2019.134558
Watson C, Paxinos G, Puelles L (2012) The mouse nervous system. Academic Press
Monickaraj F, McGuire PG, Nitta CF, Ghosh K, Das A (2016) Cathepsin D: an Mvarphi-derived factor mediating increased endothelial cell permeability with implications for alteration of the blood-retinal barrier in diabetic retinopathy. FASEB J 30(4):1670–1682. https://doi.org/10.1096/fj.15-279,802
Montague-Cardoso K, Pitcher T, Chisolm K, Salera G, Lindstrom E, Hewitt E, Solito E, Malcangio M (2020) Changes in vascular permeability in the spinal cord contribute to chemotherapy-induced neuropathic pain. Brain Behav Immun 83:248–259. https://doi.org/10.1016/j.bbi.2019.10.018
Kim J, Choi Y, Ahn M, Ekanayake P, Tanaka A, Matsuda H, Shin T (2019) Microglial and astroglial reaction in the olfactory bulb of mice after Triton X-100 application. Acta Histochem 121(5):546–552. https://doi.org/10.1016/j.acthis.2019.04.003
Brambilla R (2019) The contribution of astrocytes to the neuroinflammatory response in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol 137(5):757–783. https://doi.org/10.1007/s00401-019-01980-7
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541(7638):481–487. https://doi.org/10.1038/nature21029
Levicar N, Strojnik T, Kos J, Dewey RA, Pilkington GJ, Lah TT (2002) Lysosomal enzymes, cathepsins in brain tumour invasion. J Neuro-Oncol 58(1):21–32. https://doi.org/10.1023/a:1015892911420
Hoyk Z, Parducz A, Garcia-Segura LM (2004) Dehydroepiandrosterone regulates astroglia reaction to denervation of olfactory glomeruli. Glia 48(3):207–216. https://doi.org/10.1002/glia.20070
Nathan BP, Nisar R, Randall S, Short J, Sherrow M, Wong GK, Struble RG (2001) Apolipoprotein E is upregulated in olfactory bulb glia following peripheral receptor lesion in mice. Exp Neurol 172(1):128–136. https://doi.org/10.1006/exnr.2001.7762
Fan K, Li D, Zhang Y, Han C, Liang J, Hou C, Xiao H, Ikenaka K et al (2015) The induction of neuronal death by up-regulated microglial cathepsin H in LPS-induced neuroinflammation. J Neuroinflammation 12:54. https://doi.org/10.1186/s12974-015-0268-x
Fan K, Wu X, Fan B, Li N, Lin Y, Yao Y, Ma J (2012) Up-regulation of microglial cathepsin C expression and activity in lipopolysaccharide-induced neuroinflammation. J Neuroinflammation 9:96. https://doi.org/10.1186/1742-2094-9-96
Banerjee M, Sasse VA, Wang Y, Maulik M, Kar S (2015) Increased levels and activity of cathepsins B and D in kainate-induced toxicity. Neuroscience 284:360–373. https://doi.org/10.1016/j.neuroscience.2014.10.003
Pislar A, Bozic B, Zidar N, Kos J (2017) Inhibition of cathepsin X reduces the strength of microglial-mediated neuroinflammation. Neuropharmacology 114:88–100. https://doi.org/10.1016/j.neuropharm.2016.11.019
Lowry JR, Klegeris A (2018) Emerging roles of microglial cathepsins in neurodegenerative disease. Brain Res Bull 139:144–156. https://doi.org/10.1016/j.brainresbull.2018.02.014
Brown GC, Vilalta A (2015) How microglia kill neurons. Brain Res 1628(Pt B):288–297. https://doi.org/10.1016/j.brainres.2015.08.031
Zaidi N, Maurer A, Nieke S, Kalbacher H (2008) Cathepsin D: a cellular roadmap. Biochem Biophys Res Commun 376(1):5–9. https://doi.org/10.1016/j.bbrc.2008.08.099
Braidy N, Brew BJ, Inestrosa NC, Chung R, Sachdev P, Guillemin GJ (2014) Changes in Cathepsin D and Beclin-1 mRNA and protein expression by the excitotoxin quinolinic acid in human astrocytes and neurons. Metab Brain Dis 29(3):873–883. https://doi.org/10.1007/s11011-014-9557-9
Moon C, Kim S, Wie M, Kim H, Cheong J, Park J, Jee Y, Tanuma N et al (2000) Increased expression of p53 and Bax in the spinal cords of rats with experimental autoimmune encephalomyelitis. Neurosci Lett 289(1):41–44
Mantle D, Falkous G, Ishiura S, Perry RH, Perry EK (1995) Comparison of cathepsin protease activities in brain tissue from normal cases and cases with Alzheimer’s disease, Lewy body dementia, Parkinson’s disease and Huntington’s disease. J Neurol Sci 131(1):65–70
Kikuchi H, Yamada T, Furuya H, Doh-ura K, Ohyagi Y, Iwaki T, Kira J (2003) Involvement of cathepsin B in the motor neuron degeneration of amyotrophic lateral sclerosis. Acta Neuropathol 105(5):462–468. https://doi.org/10.1007/s00401-002-0667-9
Koike M, Shibata M, Ezaki J, Peters C, Saftig P, Kominami E, Uchiyama Y (2013) Differences in expression patterns of cathepsin C/dipeptidyl peptidase I in normal, pathological and aged mouse central nervous system. Eur J Neurosci 37(5):816–830. https://doi.org/10.1111/ejn.12096
Kingham PJ, Pocock JM (2001) Microglial secreted cathepsin B induces neuronal apoptosis. J Neurochem 76(5):1475–1484
Okada R, Zhang X, Harada Y, Wu Z, Nakanishi H (2018) Cathepsin H deficiency in mice induces excess Th1 cell activation and early-onset of EAE though impairment of toll-like receptor 3 cascade. Inflamm Res 67(5):371–374. https://doi.org/10.1007/s00011-018-1136-9
Thanei S, Theron M, Silva AP, Reis B, Branco L, Schirmbeck L, Kolb FA, Haap W et al (2017) Cathepsin S inhibition suppresses autoimmune-triggered inflammatory responses in macrophages. Biochem Pharmacol 146:151–164. https://doi.org/10.1016/j.bcp.2017.10.001
Wendt W, Lubbert H, Stichel CC (2008) Upregulation of cathepsin S in the aging and pathological nervous system of mice. Brain Res 1232:7–20. https://doi.org/10.1016/j.brainres.2008.07.067
Petanceska S, Burke S, Watson SJ, Devi L (1994) Differential distribution of messenger RNAs for cathepsins B, L and S in adult rat brain: an in situ hybridization study. Neuroscience 59(3):729–738. https://doi.org/10.1016/0306-4522(94)90190-2
Liuzzo JP, Petanceska SS, Devi LA (1999) Neurotrophic factors regulate cathepsin S in macrophages and microglia: a role in the degradation of myelin basic protein and amyloid beta peptide. Mol Med 5(5):334–343
Nakanishi H (2003) Microglial functions and proteases. Mol Neurobiol 27(2):163–176. https://doi.org/10.1385/MN:27:2:163
Hao HP, Doh-Ura K, Nakanishi H (2007) Impairment of microglial responses to facial nerve axotomy in cathepsin S-deficient mice. J Neurosci Res 85(10):2196–2206. https://doi.org/10.1002/jnr.21357
Funding
This research was supported by the National Research Foundation of Korea (Grant number: NRF-2017R1A2B4012487 and NRF-2019R1A2C1087753).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experimental procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals of Jeju National University (Permission No. 2017-0019 and 2019-0048). The animal protocols also conformed to current international laws and the policies of the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, 1985, revised 1996).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• All tested cathepsins were upregulated in the olfactory bulb of mice with EAE.
• Cathepsins were expressed at varying levels in glial cells and inflammatory cells in the olfactory bulb of EAE mice.
• Cathepsin D potentially contributes to the clearance of cell debris in the olfactory bulb of EAE mice.
• Upregulation of cathepsins in olfactory bulbs is potentially associated with olfactory dysfunction in autoimmune encephalomyelitis.
Electronic supplementary material
ESM 1
(DOCX 767 kb)
Rights and permissions
About this article
Cite this article
Kim, J., Ahn, M., Choi, Y. et al. Upregulation of Cathepsins in Olfactory Bulbs Is Associated with Transient Olfactory Dysfunction in Mice with Experimental Autoimmune Encephalomyelitis. Mol Neurobiol 57, 3412–3423 (2020). https://doi.org/10.1007/s12035-020-01952-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01952-z