Abstract
Mutations in the CDKL5 gene, which encodes a serine/threonine kinase, causes a rare encephalopathy, characterized by early-onset epilepsy and severe intellectual disability, named CDKL5 deficiency disorder (CDD). In vitro and in vivo studies in mouse models of Cdkl5 deficiency have highlighted the role of CDKL5 in brain development and, in particular, in the morphogenesis and synaptic connectivity of hippocampal and cortical neurons. Interestingly, Cdkl5 deficiency in mice increases vulnerability to excitotoxic stress in hippocampal neurons. However, the mechanism by which CDKL5 controls neuronal survival is far from being understood. To investigate further the function of CDKL5 and dissect the molecular mechanisms underlying neuronal survival, we generated a human neuronal model of CDKL5 deficiency, using CRISPR/Cas9-mediated genome editing. We demonstrated that CDKL5 deletion in human neuroblastoma SH-SY5Y cells not only impairs neuronal maturation but also reduces cell proliferation and survival, with alterations in the AKT and ERK signaling pathways and an increase in the proapoptotic BAX protein and in DNA damage-associated biomarkers (i.e., γH2AX, RAD50, and PARP1). Furthermore, CDKL5-deficient cells were hypersensitive to DNA damage-associated stress, accumulated more DNA damage foci (γH2AX positive) and were more prone to cell death than the controls. Importantly, increased kainic acid-induced cell death of hippocampal neurons of Cdkl5 KO mice correlated with an increased γH2AX immunostaining. The results suggest a previously unknown role for CDKL5 in DNA damage response that could underlie the pro-survival function of CDKL5.








Similar content being viewed by others
Change history
17 February 2020
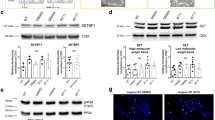
The original version of this article unfortunately contained error in Fig. 5a to where a panel is missing.
References
Fehr S, Wilson M, Downs J, Williams S, Murgia A, Sartori S, Vecchi M, Ho G et al (2013) The CDKL5 disorder is an independent clinical entity associated with early-onset encephalopathy. Eur J Hum Genet 21(3):266–273. https://doi.org/10.1038/ejhg.2012.156
Dale T, Downs J, Olson H, Bergin AM, Smith S, Leonard H (2019) Cannabis for refractory epilepsy in children: a review focusing on CDKL5 deficiency disorder. Epilepsy Res 151:31–39. https://doi.org/10.1016/j.eplepsyres.2019.02.001
Montini E, Andolfi G, Caruso A, Buchner G, Walpole SM, Mariani M, Consalez G, Trump D et al (1998) Identification and characterization of a novel serine-threonine kinase gene from the Xp22 region. Genomics 51(3):427–433. https://doi.org/10.1006/geno.1998.5391
Lin C, Franco B, Rosner MR (2005) CDKL5/Stk9 kinase inactivation is associated with neuronal developmental disorders. Hum Mol Genet 14(24):3775–3786. https://doi.org/10.1093/hmg/ddi391
Rusconi L, Salvatoni L, Giudici L, Bertani I, Kilstrup-Nielsen C, Broccoli V, Landsberger N (2008) CDKL5 expression is modulated during neuronal development and its subcellular distribution is tightly regulated by the C-terminal tail. J Biol Chem 283(44):30101–30111. https://doi.org/10.1074/jbc.M804613200
Bahi-Buisson N, Bienvenu T (2012) CDKL5-related disorders: from clinical description to molecular genetics. Mol Syndromol 2(3-5):137–152
Fehr S, Downs J, Ho G, de Klerk N, Forbes D, Christodoulou J, Williams S, Leonard H (2016) Functional abilities in children and adults with the CDKL5 disorder. Am J Med Genet A 170(11):2860–2869. https://doi.org/10.1002/ajmg.a.37851
Fehr S, Wong K, Chin R, Williams S, de Klerk N, Forbes D, Krishnaraj R, Christodoulou J et al (2016) Seizure variables and their relationship to genotype and functional abilities in the CDKL5 disorder. Neurology 87(21):2206–2213. https://doi.org/10.1212/WNL.0000000000003352
Neupauerova J, Sterbova K, Vlckova M, Sebronova V, Marikova T, Krutova M, David S, Krsek P et al (2017) Two novel variants affecting CDKL5 transcript associated with epileptic encephalopathy. Genet Test Mol Biomarkers 21(10):613–618. https://doi.org/10.1089/gtmb.2017.0110
Kilstrup-Nielsen C, Rusconi L, La Montanara P, Ciceri D, Bergo A, Bedogni F, Landsberger N (2012) What we know and would like to know about CDKL5 and its involvement in epileptic encephalopathy. Neural Plast 2012:728267. https://doi.org/10.1155/2012/728267
Amendola E, Zhan Y, Mattucci C, Castroflorio E, Calcagno E, Fuchs C, Lonetti G, Silingardi D et al (2014) Mapping pathological phenotypes in a mouse model of CDKL5 disorder. PLoS One 9(5):e91613. https://doi.org/10.1371/journal.pone.0091613
Wang IT, Allen M, Goffin D, Zhu X, Fairless AH, Brodkin ES, Siegel SJ, Marsh ED et al (2012) Loss of CDKL5 disrupts kinome profile and event-related potentials leading to autistic-like phenotypes in mice. Proc Natl Acad Sci U S A 109(52):21516–21521. https://doi.org/10.1073/pnas.1216988110
Tang S, Wang IJ, Yue C, Takano H, Terzic B, Pance K, Lee JY, Cui Y et al (2017) Loss of CDKL5 in glutamatergic neurons disrupts hippocampal microcircuitry and leads to memory impairment in mice. J Neurosci 37(31):7420–7437. https://doi.org/10.1523/JNEUROSCI.0539-17.2017
Okuda K, Takao K, Watanabe A, Miyakawa T, Mizuguchi M, Tanaka T (2018) Comprehensive behavioral analysis of the Cdkl5 knockout mice revealed significant enhancement in anxiety- and fear-related behaviors and impairment in both acquisition and long-term retention of spatial reference memory. PLoS One 13(4):e0196587. https://doi.org/10.1371/journal.pone.0196587
Fuchs C, Gennaccaro L, Trazzi S, Bastianini S, Bettini S, Lo Martire V, Ren E, Medici G et al (2018) Heterozygous CDKL5 knockout female mice are a valuable animal model for CDKL5 disorder. Neural Plast 2018:9726950. https://doi.org/10.1155/2018/9726950
Fuchs C, Rimondini R, Viggiano R, Trazzi S, De Franceschi M, Bartesaghi R, Ciani E (2015) Inhibition of GSK3beta rescues hippocampal development and learning in a mouse model of CDKL5 disorder. Neurobiol Dis 82:298–310. https://doi.org/10.1016/j.nbd.2015.06.018
Della Sala G, Putignano E, Chelini G, Melani R, Calcagno E, Michele Ratto G, Amendola E, Gross CT et al (2016) Dendritic spine instability in a mouse model of CDKL5 disorder is rescued by insulin-like growth factor 1. Biol Psychiatry 80(4):302–311. https://doi.org/10.1016/j.biopsych.2015.08.028
Ren E, Roncacé V, Trazzi S, Fuchs C, Medici G, Gennaccaro L, Loi M, Galvani G et al (2019) Functional and structural impairments in the perirhinal cortex of a mouse model of CDKL5 deficiency disorder are rescued by a TrkB agonist. Front Cell Neurosci 13(169). https://doi.org/10.3389/fncel.2019.00169
Pizzo R, Gurgone A, Castroflorio E, Amendola E, Gross C, Sassoe-Pognetto M, Giustetto M (2016) Lack of Cdkl5 disrupts the organization of excitatory and inhibitory synapses and parvalbumin interneurons in the primary visual cortex. Front Cell Neurosci 10:261. https://doi.org/10.3389/fncel.2016.00261
Ricciardi S, Ungaro F, Hambrock M, Rademacher N, Stefanelli G, Brambilla D, Sessa A, Magagnotti C et al (2012) CDKL5 ensures excitatory synapse stability by reinforcing NGL-1-PSD95 interaction in the postsynaptic compartment and is impaired in patient iPSC-derived neurons. Nat Cell Biol 14(9):911–923. https://doi.org/10.1038/ncb2566
Trazzi S, De Franceschi M, Fuchs C, Bastianini S, Viggiano R, Lupori L, Mazziotti R, Medici G et al (2018) CDKL5 protein substitution therapy rescues neurological phenotypes of a mouse model of CDKL5 disorder. Hum Mol Genet 27(9):1572–1592. https://doi.org/10.1093/hmg/ddy064
Zhu YC, Xiong ZQ (2019) Molecular and synaptic bases of CDKL5 disorder. Dev Neurobiol 79(1):8–19. https://doi.org/10.1002/dneu.22639
Fuchs C, Medici G, Trazzi S, Gennaccaro L, Galvani G, Berteotti C, Ren E, Loi M et al (2019) CDKL5 deficiency predisposes neurons to cell death through the deregulation of SMAD3 signaling. Brain Pathol. https://doi.org/10.1111/bpa.12716
Strober W (2015) Trypan blue exclusion test of cell viability. Curr Protoc Immunol 111:A3 B 1–A3 B 3. https://doi.org/10.1002/0471142735.ima03bs111
Valli E, Trazzi S, Fuchs C, Erriquez D, Bartesaghi R, Perini G, Ciani E (2012) CDKL5, a novel MYCN-repressed gene, blocks cell cycle and promotes differentiation of neuronal cells. Biochim Biophys Acta 1819(11–12):1173–1185. https://doi.org/10.1016/j.bbagrm.2012.08.001
Takahashi T, Nowakowski RS, Caviness VS Jr (1993) Cell cycle parameters and patterns of nuclear movement in the neocortical proliferative zone of the fetal mouse. J Neurosci 13(2):820–833
Li Z, Lin H, Zhu Y, Wang M, Luo J (2001) Disruption of cell cycle kinetics and cyclin-dependent kinase system by ethanol in cultured cerebellar granule progenitors. Brain Res Dev Brain Res 132(1):47–58
Fujita S (1967) Quantitative analysis of cell proliferation and differentiation in the cortex of the postnatal mouse cerebellum. J Cell Biol 32(2):277–287
Contestabile A, Fila T, Bartesaghi R, Ciani E (2009) Cell cycle elongation impairs proliferation of cerebellar granule cell precursors in the Ts65Dn mouse, an animal model for Down syndrome. Brain Pathol 19(2):224–237. https://doi.org/10.1111/j.1750-3639.2008.00168.x
Trazzi S, Fuchs C, Viggiano R, De Franceschi M, Valli E, Jedynak P, Hansen FK, Perini G et al (2016) HDAC4: a key factor underlying brain developmental alterations in CDKL5 disorder. Hum Mol Genet 25(18):3887–3907. https://doi.org/10.1093/hmg/ddw231
Contestabile A, Fila T, Cappellini A, Bartesaghi R, Ciani E (2009) Widespread impairment of cell proliferation in the neonate Ts65Dn mouse, a model for Down syndrome. Cell Prolif 42(2):171–181. https://doi.org/10.1111/j.1365-2184.2009.00587.x
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Wu G, Lu ZH, Wang J, Wang Y, Xie X, Meyenhofer MF, Ledeen RW (2005) Enhanced susceptibility to kainate-induced seizures, neuronal apoptosis, and death in mice lacking gangliotetraose gangliosides: protection with LIGA 20, a membrane-permeant analog of GM1. J Neurosci 25(47):11014–11022. https://doi.org/10.1523/JNEUROSCI.3635-05.2005
Contestabile A, Fila T, Ceccarelli C, Bonasoni P, Bonapace L, Santini D, Bartesaghi R, Ciani E (2007) Cell cycle alteration and decreased cell proliferation in the hippocampal dentate gyrus and in the neocortical germinal matrix of fetuses with Down syndrome and in Ts65Dn mice. Hippocampus 17(8):665–678. https://doi.org/10.1002/hipo.20308
Encinas M, Iglesias M, Liu Y, Wang H, Muhaisen A, Cena V, Gallego C, Comella JX (2000) Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J Neurochem 75(3):991–1003
Christensen J, Steain M, Slobedman B, Abendroth A (2011) Differentiated neuroblastoma cells provide a highly efficient model for studies of productive varicella-zoster virus infection of neuronal cells. J Virol 85(16):8436–8442. https://doi.org/10.1128/JVI.00515-11
Gimenez-Cassina A, Lim F, Diaz-Nido J (2006) Differentiation of a human neuroblastoma into neuron-like cells increases their susceptibility to transduction by herpesviral vectors. J Neurosci Res 84(4):755–767. https://doi.org/10.1002/jnr.20976
Shipley MM, Mangold CA, Kuny CV, Szpara ML (2017) Differentiated human SH-SY5Y cells provide a reductionist model of herpes simplex virus 1 neurotropism. J Virol 91(23). https://doi.org/10.1128/JVI.00958-17
Fuchs C, Trazzi S, Torricella R, Viggiano R, De Franceschi M, Amendola E, Gross C, Calza L et al (2014) Loss of CDKL5 impairs survival and dendritic growth of newborn neurons by altering AKT/GSK-3beta signaling. Neurobiol Dis 70:53–68. https://doi.org/10.1016/j.nbd.2014.06.006
Kaplan DR, Matsumoto K, Lucarelli E, Thiele CJ (1993) Induction of TrkB by retinoic acid mediates biologic responsiveness to BDNF and differentiation of human neuroblastoma cells. Eukaryotic Signal Transduction Group. Neuron 11(2):321–331
Ferrari-Toninelli G, Bonini SA, Uberti D, Napolitano F, Stante M, Santoro F, Minopoli G, Zambrano N et al (2009) Notch activation induces neurite remodeling and functional modifications in SH-SY5Y neuronal cells. Dev Neurobiol 69(6):378–391. https://doi.org/10.1002/dneu.20710
Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF (2015) Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res 35(6):600–604. https://doi.org/10.3109/10799893.2015.1030412
Sanchez-Alegria K, Flores-Leon M, Avila-Munoz E, Rodriguez-Corona N, Arias C (2018) PI3K signaling in neurons: a central node for the control of multiple functions. Int J Mol Sci 19(12). https://doi.org/10.3390/ijms19123725
Fuchs C, Fustini N, Trazzi S, Gennaccaro L, Rimondini R, Ciani E (2018) Treatment with the GSK3-beta inhibitor Tideglusib improves hippocampal development and memory performance in juvenile, but not adult, Cdkl5 knockout mice. Eur J Neurosci 47(9):1054–1066. https://doi.org/10.1111/ejn.13923
Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD (1997) Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106(6):348–360
Fielder E, von Zglinicki T, Jurk D (2017) The DNA damage response in neurons: die by apoptosis or survive in a senescence-like state? J Alzheimers Dis 60(s1):S107–S131. https://doi.org/10.3233/JAD-161221
Ji J, Zhang Y, Redon CE, Reinhold WC, Chen AP, Fogli LK, Holbeck SL, Parchment RE et al (2017) Phosphorylated fraction of H2AX as a measurement for DNA damage in cancer cells and potential applications of a novel assay. PLoS One 12(2):e0171582. https://doi.org/10.1371/journal.pone.0171582
Podhorecka M, Skladanowski A, Bozko P (2010, 2010) H2AX phosphorylation: its role in DNA damage response and cancer therapy. J Nucleic Acids. https://doi.org/10.4061/2010/920161
Althaus FR, Richter C (1987) ADP-ribosylation of proteins. Enzymology and biological significance. Mol Biol Biochem Biophys 37:1–237
Cleaver JE, Morgan WF (1991) Poly(ADP-ribose)polymerase: a perplexing participant in cellular responses to DNA breakage. Mutat Res 257(1):1–18
Lindahl T, Satoh MS, Poirier GG, Klungland A (1995) Post-translational modification of poly(ADP-ribose) polymerase induced by DNA strand breaks. Trends Biochem Sci 20(10):405–411
Rancourt A, Satoh MS (2009) Delocalization of nucleolar poly(ADP-ribose) polymerase-1 to the nucleoplasm and its novel link to cellular sensitivity to DNA damage. DNA Repair (Amst) 8(3):286–297. https://doi.org/10.1016/j.dnarep.2008.11.018
Meder VS, Boeglin M, de Murcia G, Schreiber V (2005) PARP-1 and PARP-2 interact with nucleophosmin/B23 and accumulate in transcriptionally active nucleoli. J Cell Sci 118(Pt 1):211–222. https://doi.org/10.1242/jcs.01606
Uutela M, Lindholm J, Rantamaki T, Umemori J, Hunter K, Voikar V, Castren ML (2014) Distinctive behavioral and cellular responses to fluoxetine in the mouse model for Fragile X syndrome. Front Cell Neurosci 8:150. https://doi.org/10.3389/fncel.2014.00150
Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT (1989) Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 2(6):1547–1558
Yang JL, Sykora P, Wilson DM 3rd, Mattson MP, Bohr VA (2011) The excitatory neurotransmitter glutamate stimulates DNA repair to increase neuronal resiliency. Mech Ageing Dev 132(8–9):405–411. https://doi.org/10.1016/j.mad.2011.06.005
Narciso L, Parlanti E, Racaniello M, Simonelli V, Cardinale A, Merlo D, Dogliotti E (2016) The response to oxidative DNA damage in neurons: mechanisms and disease. Neural Plast 2016:3619274. https://doi.org/10.1155/2016/3619274
Altman BJ, Rathmell JC (2012) Metabolic stress in autophagy and cell death pathways. Cold Spring Harb Perspect Biol 4(9):a008763. https://doi.org/10.1101/cshperspect.a008763
Liu J, Kim J, Oberdoerffer P (2013) Metabolic modulation of chromatin: implications for DNA repair and genomic integrity. Front Genet 4:182. https://doi.org/10.3389/fgene.2013.00182
Chen Q, Zhu YC, Yu J, Miao S, Zheng J, Xu L, Zhou Y, Li D et al (2010) CDKL5, a protein associated with rett syndrome, regulates neuronal morphogenesis via Rac1 signaling. J Neurosci 30(38):12777–12786. https://doi.org/10.1523/JNEUROSCI.1102-10.2010
Luo J (2012) The role of GSK3beta in the development of the central nervous system. Front Biol 7(3):212–220. https://doi.org/10.1007/s11515-012-1222-2
Verma N, Franchitto M, Zonfrilli A, Cialfi S, Palermo R, Talora C (2019) DNA damage stress: cui prodest? Int J Mol Sci 20(5). https://doi.org/10.3390/ijms20051073
Wang JY (2001) DNA damage and apoptosis. Cell Death Differ 8(11):1047–1048. https://doi.org/10.1038/sj.cdd.4400938
Zinkel S, Gross A, Yang E (2006) BCL2 family in DNA damage and cell cycle control. Cell Death Differ 13(8):1351–1359. https://doi.org/10.1038/sj.cdd.4401987
Barbiero I, Valente D, Chandola C, Magi F, Bergo A, Monteonofrio L, Tramarin M, Fazzari M et al (2017) CDKL5 localizes at the centrosome and midbody and is required for faithful cell division. Sci Rep 7(1):6228. https://doi.org/10.1038/s41598-017-05875-z
Enriquez-Rios V, Dumitrache LC, Downing SM, Li Y, Brown EJ, Russell HR, McKinnon PJ (2017) DNA-PKcs, ATM, and ATR interplay maintains genome integrity during neurogenesis. J Neurosci 37(4):893–905. https://doi.org/10.1523/JNEUROSCI.4213-15.2016
Kinoshita Y, Wenzel HJ, Kinoshita C, Schwartzkroin PA, Morrison RS (2012) Acute, but reversible, kainic acid-induced DNA damage in hippocampal CA1 pyramidal cells of p53-deficient mice. Epilepsia 53(Suppl 1):125–133. https://doi.org/10.1111/j.1528-1167.2012.03483.x
Youngsoo L, Inseo C, Jusik K, Keeeun K (2016) DNA damage to human genetic disorders with neurodevelopmental defects. J Genet Med 13(1):1–13
Markkanen E, Meyer U, Dianov GL (2016) DNA damage and repair in schizophrenia and autism: implications for cancer comorbidity and beyond. Int J Mol Sci 17(6). https://doi.org/10.3390/ijms17060856
Acknowledgments
This work was supported by the Italian parent association “CDKL5 insieme verso la cura.”
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 541 kb)
Rights and permissions
About this article
Cite this article
Loi, M., Trazzi, S., Fuchs, C. et al. Increased DNA Damage and Apoptosis in CDKL5-Deficient Neurons. Mol Neurobiol 57, 2244–2262 (2020). https://doi.org/10.1007/s12035-020-01884-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01884-8