Abstract
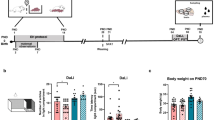
Exposure to adverse events during gestation has detrimental effects on the maturation of specific brain networks, triggering changes in the expression of several stress-related mechanisms that may lead to long-lasting functional consequences, including cognitive deterioration. On these bases, the aim of the present study was to investigate the effects of early-life stress exposure on cognition and to explore potential molecular mechanisms contributing to the long-term functional impairment. We found that exposure to prenatal stress, a well-established animal model of early-life adversity, produces a significant disruption in the novel object recognition test both in male and female adult rats, although such impairment was more pronounced in females. Furthermore, the cognitive dysfunction observed during the behavioral test appears to be sustained by a disrupted activation of key networks of genes that may be required for proper cognitive performance. In particular, within the dorsal hippocampus, a brain region critical for cognition, the glucocorticoid, the inflammatory, and the protein kinase A signaling pathways are regulated by the novel object recognition test in an opposite manner in animals previously exposed to prenatal stress, when compared with control animals. These data further support the evidence that early-life stress exposure prompts cognitive impairment and suggest that this is the consequence of inability to activate the proper transcriptional machinery required for the cognitive performance.






Similar content being viewed by others
References
Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, Reyes TM et al (2010) Early life programming and neurodevelopmental disorders. Biol Psychiatry 68:314–319. https://doi.org/10.1016/j.biopsych.2010.05.028
Heim C, Plotsky PM, Nemeroff CB (2004) Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology 29:641–648. https://doi.org/10.1038/sj.npp.1300397
Provençal N, Binder EB (2015) The effects of early life stress on the epigenome: from the womb to adulthood and even before. Exp Neurol 268:10–20. https://doi.org/10.1016/j.expneurol.2014.09.001
Duman RS, Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127. https://doi.org/10.1016/j.biopsych.2006.02.013
Fumagalli F, Molteni R, Racagni G, Riva MA (2007) Stress during development: impact on neuroplasticity and relevance to psychopathology. Prog Neurobiol 81:197–217. https://doi.org/10.1016/j.pneurobio.2007.01.002
Harris A, Seckl J (2011) Glucocorticoids, prenatal stress and the programming of disease. Horm Behav 59:279–289. https://doi.org/10.1016/j.yhbeh.2010.06.007
Andersen SL (2003) Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev 27:3–18. https://doi.org/10.1016/S0149-7634(03)00005-8
Nawaz A, Batool Z, Shazad S, Rafiq S, Afzal A, Haider S (2018) Physical enrichment enhances memory function by regulating stress hormone and brain acetylcholinesterase activity in rats exposed to restraint stress. Life Sci 207:42–49. https://doi.org/10.1016/j.lfs.2018.05.049
Cattaneo A, Riva MA (2016) Stress-induced mechanisms in mental illness: a role for glucocorticoid signalling. J Steroid Biochem Mol Biol 160:169–174. https://doi.org/10.1016/j.jsbmb.2015.07.021
Association American Psychiatric (2013) Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC
Darnaudéry M, Maccari S (2008) Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res Rev 57:571–585. https://doi.org/10.1016/j.brainresrev.2007.11.004
Luoni A, Berry A, Calabrese F, Capoccia S, Bellisario V, Gass P, Cirulli F, Riva MA (2014) Delayed BDNF alterations in the prefrontal cortex of rats exposed to prenatal stress: preventive effect of lurasidone treatment during adolescence. Eur Neuropsychopharmacol 24:986–995. https://doi.org/10.1016/j.euroneuro.2013.12.010
Luoni A, Berry A, Raggi C, Bellisario V, Cirulli F, Riva MA (2016) Sex-specific effects of prenatal stress on Bdnf expression in response to an acute challenge in rats: a role for Gadd45β. Mol Neurobiol 53:7037–7047. https://doi.org/10.1007/s12035-015-9569-4
Maccari S, Krugers HJ, Morley-Fletcher S, Szyf M, Brunton PJ (2014) The consequences of early-life adversity: neurobiological, behavioural and epigenetic adaptations. J Neuroendocrinol 26:707–723. https://doi.org/10.1111/jne.12175
Begni V, Riva MA, Cattaneo A (2017) Cellular and molecular mechanisms of the brain-derived neurotrophic factor in physiological and pathological conditions. Clin Sci 131:123–138. https://doi.org/10.1042/CS20160009
Cowansage KK, LeDoux JE, Monfils MH (2010) Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr Mol Pharmacol 3:12–29. https://doi.org/10.2174/1874467211003010012
Iosifescu DV (2012) The relation between mood, cognition and psychosocial functioning in psychiatric disorders. Eur Neuropsychopharmacol 22(Suppl 3):S499–S504. https://doi.org/10.1016/j.euroneuro.2012.08.002
Zhang H, Shang Y, Xiao X, Yu M, Zhang T (2017) Prenatal stress-induced impairments of cognitive flexibility and bidirectional synaptic plasticity are possibly associated with autophagy in adolescent male-offspring. Exp Neurol 298:68–78. https://doi.org/10.1016/j.expneurol.2017.09.001
Zeng Y, Brydges NM, Wood ER, Drake AJ, Hall J (2015) Prenatal glucocorticoid exposure in rats: programming effects on stress reactivity and cognition in adult offspring. Stress 18:353–361. https://doi.org/10.3109/10253890.2015.1055725
Nazeri M, Shabani M, Ghotbi Ravandi S, Aghaei I, Nozari M, Mazhari S (2015) Psychological or physical prenatal stress differentially affects cognition behaviors. Physiol Behav 142:155–160. https://doi.org/10.1016/j.physbeh.2015.02.016
Barzegar M, Sajjadi FS, Talaei SA, Hamidi G, Salami M (2015) Prenatal exposure to noise stress: anxiety, impaired spatial memory, and deteriorated hippocampal plasticity in postnatal life. Hippocampus 25:187–196. https://doi.org/10.1002/hipo.22363
Rice CJ, Sandman CA, Lenjavi MR, Baram TZ (2008) A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 149:4892–4900. https://doi.org/10.1210/en.2008-0633
Yang J, Hou C, Ma N, Liu J, Zhang Y, Zhou J, Xu L, Li L (2007) Enriched environment treatment restores impaired hippocampal synaptic plasticity and cognitive deficits induced by prenatal chronic stress. Neurobiol Learn Mem 87:257–263. https://doi.org/10.1016/j.nlm.2006.09.001
Dardou D, Datiche F, Cattarelli M (2008) Memory is differently impaired during aging according to the learning tasks in the rat. Behav Brain Res 194:193–200. https://doi.org/10.1016/j.bbr.2008.07.007
Realini N, Vigano’ D, Guidali C, Zamberletti E, Rubino T, Parolaro D (2011) Chronic URB597 treatment at adulthood reverted most depressive-like symptoms induced by adolescent exposure to THC in female rats. Neuropharmacology 60:235–243. https://doi.org/10.1016/j.neuropharm.2010.09.003
Zamberletti E, Prini P, Speziali S, Gabaglio M, Solinas M, Parolaro D, Rubino T (2012) Gender-dependent behavioral and biochemical effects of adolescent delta-9-tetrahydrocannabinol in adult maternally deprived rats. Neuroscience 204:245–257. https://doi.org/10.1016/j.neuroscience.2011.11.038
Cattaneo A, Cattane N, Malpighi C, Czamara D, Suarez A, Mariani N, Kajantie E, Luoni A et al (2018) FoxO1, A2M, and TGF-β1: three novel genes predicting depression in gene X environment interactions are identified using cross-species and cross-tissues transcriptomic and miRNomic analyses. Mol Psychiatry 23:2192–2208. https://doi.org/10.1038/s41380-017-0002-4
Bolstad BM, Irizarry RA, Astrand M, Speed TP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19:185–193
Fanselow MS, Dong HW (2010) Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65:7–19. https://doi.org/10.1016/j.neuron.2009.11.031
Say GN, Karabekiroğlu K, Babadağı Z, Yüce M (2016) Maternal stress and perinatal features in autism and attention deficit/hyperactivity disorder. Pediatr Int 58:265–269. https://doi.org/10.1111/ped.12822
Scheinost D, Sinha R, Cross SN, Kwon SH, Sze G, Constable RT, Ment LR (2017) Does prenatal stress alter the developing connectome? Pediatr Res 81:214–226. https://doi.org/10.1038/pr.2016.197
Van den Bergh BRH, van den Heuvel MI, Lahti M et al (2017) Prenatal developmental origins of behavior and mental health: the influence of maternal stress in pregnancy. Neurosci Biobehav Rev S0149-7634:30734–30735. https://doi.org/10.1016/j.neubiorev.2017.07.003
Laloux C, Mairesse J, Van Camp G et al (2012) Anxiety-like behaviour and associated neurochemical and endocrinological alterations in male pups exposed to prenatal stress. Psychoneuroendocrinology 37:1646–1658. https://doi.org/10.1016/j.psyneuen.2012.02.010
Richetto J, Riva MA (2014) Prenatal maternal factors in the development of cognitive impairments in the offspring. J Reprod Immunol 104–105:20–25. https://doi.org/10.1016/j.jri.2014.03.005
Luoni A, Massart R, Nieratschker V, Nemoda Z, Blasi G, Gilles M, Witt SH, Suderman MJ et al (2016) Ankyrin-3 as a molecular marker of early-life stress and vulnerability to psychiatric disorders. Transl Psychiatry 6:e943. https://doi.org/10.1038/tp.2016.211
Fumagalli F, Bedogni F, Perez J, Racagni G, Riva MA (2004) Corticostriatal brain-derived neurotrophic factor dysregulation in adult rats following prenatal stress. Eur J Neurosci 20:1348–1354. https://doi.org/10.1111/j.1460-9568.2004.03592.x
Weinstock M (2005) The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun 19:296–308. https://doi.org/10.1016/j.bbi.2004.09.006
Anacker C, Cattaneo A, Luoni A, Musaelyan K, Zunszain PA, Milanesi E, Rybka J, Berry A et al (2013) Glucocorticoid-related molecular signalling pathways regulating hippocampal neurogenesis. Neuropsychopharmacology 38:872–883. https://doi.org/10.1038/npp.2012.253
Ulrich-Lai YM, Herman JP (2009) Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10:397–409. https://doi.org/10.1038/nrn2647
Kelleher RJ, Govindarajan A, Jung HY et al (2004) Translational control by MAPK signalling in long-term synaptic plasticity and memory. Cell 116:467–479. https://doi.org/10.1016/S0092-8674(04)00115-1
Kang H, Sun LD, Atkins CM, Soderling TR, Wilson MA, Tonegawa S (2001) An important role of neural activity-dependent CaMKIV signalling in the consolidation of long-term memory. Cell 106:771–783. https://doi.org/10.1016/S0092-8674(01)00497-4
Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER (1999) A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell 99:221–237. https://doi.org/10.1016/S0092-8674(00)81653-0
Guan L, Jia N, Zhao X, Zhang X, Tang G, Yang L, Sun H, Wang D et al (2013) The involvement of ERK/CREB/Bcl-2 in depression-like behavior in prenatally stressed offspring rats. Brain Res Bull 99:1–8. https://doi.org/10.1016/j.brainresbull.2013.08.003
Lian S, Wang D, Xu B, Guo W, Wang L, Li W, Ji H, Wang J et al (2018) Prenatal cold stress: Effect on maternal hippocampus and offspring behavior in rats. Behav Brain Res 346:1–10. https://doi.org/10.1016/j.bbr.2018.02.002
Zhu Z, Sun H, Gong X, Li H (2016) Different effects of prenatal stress on ERK2/CREB/Bcl-2 expression in the hippocampus and the prefrontal cortex of adult offspring rats. Neuroreport 27:600–604. https://doi.org/10.1097/WNR.0000000000000581
Calabrese F, Brivio P, Gruca P, Lason-Tyburkiewicz M, Papp M, Riva MA (2017) Chronic mild stress-induced alterations of local protein synthesis: a role for cognitive impairment. ACS Chem Neurosci 8:817–825. https://doi.org/10.1021/acschemneuro.6b00392
Bosch OJ, Müsch W, Bredewold R, Slattery DA, Neumann ID (2007) Prenatal stress increases HPA axis activity and impairs maternal care in lactating female offspring: implications for postpartum mood disorder. Psychoneuroendocrinology 32:267–278. https://doi.org/10.1016/j.psyneuen.2006.12.012
Acknowledgements
We thank Francesca Calabrese for her contribution in the initial part of the work.
Funding
This work has been supported by the Italian Ministry of Instruction, University and Research (PRIN grant number 2015SKN9YT), Fondazione CARIPLO (grant number 2012-0503), and European Union/NEURON-ERANET to M.A.R. Moreover, A.C. received support from Ricerca Corrente (Ministry of Health) and from a NEURON-ERANET grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal experiments were conducted according to the authorization from the Health Ministry n. 295/2012-A (20/12/2012), in full accordance with the Italian legislation on animal experimentation (Decreto Legislativo 116/92) and adherent to EU recommendation (EEC Council Directive 86/609), in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
All efforts were made to minimize animal suffering and reduce the total number of animals used, while maintaining statistically valid group numbers.
Conflict of Interest
The author M.A.R. has received compensation as speaker/consultant from Lundbeck, Otzuka, Dainippon Sumitomo Pharma, and Sunovion, and he has received research grants from Lundbeck, Dainippon Sumitomo Pharma, and Sunovion. All the other authors declare that they have no financial interest or potential conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 279 kb)
Rights and permissions
About this article
Cite this article
Cattaneo, A., Begni, V., Malpighi, C. et al. Transcriptional Signatures of Cognitive Impairment in Rat Exposed to Prenatal Stress. Mol Neurobiol 56, 6251–6260 (2019). https://doi.org/10.1007/s12035-019-1523-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1523-4