Abstract
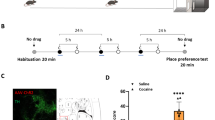
Dopamine (DA) neurons in the ventral tegmental area (VTA) are well-known components of the brain involved in reward-related behaviors and participate in the generation of new memories. Much attention has been focused to understand how DA neurons integrate a diversity of afferent signals with local excitatory and inhibitory influences regulated by somatodendritic release of dopamine. However, the mechanisms that actively forget rewarding information are still terra incognita. Using rodents in the conditioned place preference (CPP) behavioral task, we show that during acquisition D1-type DA receptors (D1R) in the VTA are crucial components of a neural circuit involving the hippocampus that induces active forgetting of cocaine-associated long-term memory, while VTA and nucleus accumbens (NAc) D1R are required for its formation. Inhibition of VTA D1R results in increased activation of VTA ERK1/2 and in prolonging memory storage of cocaine-place association in an ERK-dependent manner. Moreover, intra-VTA infusion of a specific D1 agonist induces forgetting of cocaine-associated consolidated memory. In contrast, D1R in the NAc shell, medial prefrontal cortex, or amygdala appear not to participate in the maintenance of cocaine-associated memory. Our present results suggest that at the moment of learning D1R-mediated neurotransmission in the VTA actively participates in at least two processes affecting the fate of appetitive memory: its consolidation involving NAc shell DA neurotransmission and its forgetting via DA activation of the hippocampus.





Similar content being viewed by others
References
Schultz RT, Klin A, Lombroso (2002) Genetics of childhood disorders: XLIII. Autism, Part 2: Neural foundations. J Am Acad Child Adolesc Psychiatry 41:1259–1262. https://doi.org/10.1097/00004583-200210000-00018
Wise RA (2004) Rewards wanted: molecular mechanisms of motivation. Discov Med 4:180–186
Rossato JI, Bevilaqua LRM, Izquierdo I, Medina JH, Cammarota M (2009) Dopamine controls persistence of long-term memory storage. Science (80- ) 325:1017–1020. https://doi.org/10.1126/science.1172545
Salamone JD, Correa M (2012) The mysterious motivational functions of mesolimbic dopamine. Neuron 76:470–485. https://doi.org/10.1016/j.neuron.2012.10.021
Adell A, Artigas F (2004) The somatodendritic release of dopamine in the ventral tegmental area and its regulation by afferent transmitter systems. Neurosci Biobehav Rev 28:415–431. https://doi.org/10.1016/j.neubiorev.2004.05.001
Rice ME, Patel JC (2015) Somatodendritic dopamine release: recent mechanistic insights. Philos Trans R Soc B Biol Sci 370:1–14. https://doi.org/10.1098/rstb.2014.0185
Kalivas PW, Duffy P (1989) Similar effects of daily cocaine and stress on mesocorticolimbic dopamine neurotransmission in the rat. Biol Psychiatry 25:913–928. https://doi.org/10.1016/0006-3223(89)90271-0
Sunahara RK, Guan HC, O’Dowd BF, Seeman P, Laurier LG, Ng G, George SR et al. (1991) Cloning of the gene for a human dopamine D5receptor with higher affinity for dopamine than D1. Nature 350:614–619. https://doi.org/10.1038/350614a0
Lu XY, Churchill L, Kalivas PW (1997) Expression of D1 receptor mRNA in projections from the forebrain to the ventral tegmental area. Synapse 25:205–214. https://doi.org/10.1002/(SICI)1098-2396(199702)25:2<205::AID-SYN11>3.0.CO;2-X
Harrison MB, Wiley RG, Wooten GF (1990) Selective localization of striatal D1receptors to striatonigral neurons. Brain Res 528:317–322. https://doi.org/10.1016/0006-8993(90)91674-6
Kalivas PW, Duffy P (1995) D1 receptors modulate tegmental area glutamate transmission in the ventral tegmental area. J Neurosci 15:5379–5388
Kramar CP, Chefer VI, Wise RA, Medina JH, Barbano MF (2014) Dopamine in the dorsal hippocampus impairs the late consolidation of cocaine-associated memory. Neuropsychopharmacology 39:1645–1653. https://doi.org/10.1038/npp.2014.11
Majchrzak M, Di Scala G (2000) Gaba and muscimol as reversible inactivation tools in learning and memory. [Review] [75 refs]. Neural Plast 7:19–29
Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates, 6th edn. Academic Press, New York
Katche C, Medina JH (2017) Requirement of an early activation of BDNF/c-Fos cascade in the retrosplenial cortex for the persistence of a long-lasting aversive memory. Cereb Cortex 27:1060–1067. https://doi.org/10.1093/cercor/bhv284
Undieh AS (2010) Pharmacology of signaling induced by dopamine D1-like receptor activation. Pharmacol Ther 128:37–60. https://doi.org/10.1016/j.pharmthera.2010.05.003
Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, Izquierdo I, Medina JH (2008) BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci 105:2711–2716. https://doi.org/10.1073/pnas.0711863105
Eckel-mahan KL, Phan T, Han S, Wang H, Chan GC, Scheiner ZS, Storm DR (2009) Circadian oscillation of hippocampal MAPK activity and cAmp: implications for memory persistence. 11:1074–1082
Ungless MA, Whistler JL, Malenka RC, Bonci A (2001) Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411:583–587. https://doi.org/10.1038/35079077
Di Chiara G (2002) Nucleus accumbens shell and core dopamine: Differential role in behavior and addiction. Behav Brain Res 137:75–114. https://doi.org/10.1016/S0166-4328(02)00286-3
Wise RA (2004) Dopamine, learning and motivation. Nat Rev Neurosci 5:483–494. https://doi.org/10.1038/nrn1406
Wixted JT (2004) The psychology and neuroscience of forgetting. Annu Rev Psychol 55:235–269. https://doi.org/10.1146/annurev.psych.55.090902.141555
Hardt O, Nader K, Nadel L (2013) Decay happens: the role of active forgetting in memory. Trends Cogn Sci 17:111–120. https://doi.org/10.1016/j.tics.2013.01.001
Davis RL, Zhong Y (2017) The biology of forgetting—a perspective. Neuron 95:490–503. https://doi.org/10.1016/j.neuron.2017.05.039
Frankland PW, Stefan K, Josselyn S (2013) Hippocampal neurogenesis and forgetting. Hippocampus from Cells to Syst Struct Connect Funct Contrib to Mem Flex Cogn 95–121. https://doi.org/10.1007/978-3-319-50406-3_4
Medina JH (2018) Neural, cellular and molecular mechanisms of active forgetting. Front Syst Neurosci 12:1–10. https://doi.org/10.3389/fnsys.2018.00003
Shuai Y, Lu B, Hu Y, Wang L, Sun K, Zhong Y (2010) Forgetting is regulated through Rac activity in Drosophila. Cell 140:579–589. https://doi.org/10.1016/j.cell.2009.12.044
Berry JA, Cervantes-Sandoval I, Nicholas EP, Davis RL (2012) Dopamine is required for learning and forgetting in Drosophila. Neuron 74:530–542. https://doi.org/10.1016/j.neuron.2012.04.007
Zhang X, Li Q, Wang L, Liu ZJ, Zhong Y (2016) Cdc42-dependent forgetting regulates repetition effect in prolonging memory retention. Cell Rep 16:817–825. https://doi.org/10.1016/j.celrep.2016.06.041
Himmelreich S, Masuho I, Berry JA, MacMullen C, Skamangas NK, Martemyanov KA, Davis RL (2017) Dopamine receptor DAMB signals via Gq to mediate forgetting in Drosophila. Cell Rep 21:2074–2081. https://doi.org/10.1016/j.celrep.2017.10.108
Shuai Y, Hirokawa A, Ai Y, Zhang M, Li W, Zhong Y (2015) Dissecting neural pathways for forgetting in Drosophila olfactory aversive memory. Proc Natl Acad Sci 112:E6663–E6672. https://doi.org/10.1073/pnas.1512792112
Cervantes-Sandoval I, Chakraborty M, MacMullen C, Davis RL (2016) Scribble scaffolds a signalosome for active forgetting. Neuron 90:1230–1242. https://doi.org/10.1016/j.neuron.2016.05.010
Kim Y-C, Lee H-G, Han K-A (2007) D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J Neurosci 27:7640–7647. https://doi.org/10.1523/JNEUROSCI.1167-07.2007
Takeuchi T, Duszkiewicz AJ, Sonneborn A, Spooner PA, Yamasaki M, Watanabe M, Smith CC, Fernández G et al. (2016) Locus coeruleus and dopaminergic consolidation of everyday memory. 537:357–362. https://doi.org/10.1038/nature19325.Locus
Mcnamara CG, Dupret D (2017) Two sources of dopamine for the hippocampus. Trends Neurosci 40:383–384. https://doi.org/10.1016/j.tins.2017.05.005
Ramos M, Goñi-Allo B, Aguirre N (2005) Administration of SCH 23390 into the medial prefrontal cortex blocks the expression of MDMA-induced behavioral sensitization in rats: An effect mediated by 5-HT2C receptor stimulation and not by D1 receptor blockade. Neuropsychopharmacology 30:2180–2191. https://doi.org/10.1038/sj.npp.1300735
Higgins GA, Sellers EM, Fletcher PJ (2013) From obesity to substance abuse: therapeutic opportunities for 5-HT2Creceptor agonists. Trends Pharmacol Sci 34:560–570. https://doi.org/10.1016/j.tips.2013.08.001
Harvey-Lewis C, Li Z, Higgins GA, Fletcher PJ (2016) The 5-HT2Creceptor agonist lorcaserin reduces cocaine self-administration, reinstatement of cocaine-seeking and cocaine induced locomotor activity. Neuropharmacology 101:237–245. https://doi.org/10.1016/j.neuropharm.2015.09.028
Herkenham M, Lynn AB, Decosta BR, Richfield EK (1991) Neuronal localizatin of cannabinoid receptors in the basal ganglia of the rat. Brain Res 547:267–274
Michaeli A, Yaka R (2010) Dopamine inhibits GABAA currents in ventral tegmental area dopamine neurons via activation of presynaptic G-protein coupled inwardly-rectifying potassium channels. Neuroscience 165:1159–1169. https://doi.org/10.1016/j.neuroscience.2009.11.045
Galaj E, Manuszak M, Arastehmanesh D, Ranaldi R (2014) Microinjections of a dopamine D1 receptor antagonist into the ventral tegmental area block the expression of cocaine conditioned place preference in rats. Behav Brain Res 272:279–285. https://doi.org/10.1016/j.bbr.2014.07.008
Bellone C, Lüscher C (2006) Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci 9:636–641. https://doi.org/10.1038/nn1682
Steketee JD (1998) Repeated injection of GBR 12909 , but not cocaine or WIN 35 , 065-2 , into the ventral tegmental area induces behavioral sensitization. Behav Brain Res 97:39–48. https://doi.org/10.1016/S0166-4328
Steketee JD, Braswell B (1997) Injection of SCH 23390, but not 7-hydroxy-DPAT, into the ventral tegmental area blocks the acute motor-stimulant response to cocaine. Behav Pharmacol 8:58–64. https://doi.org/10.1097/00008877
Valjent E, Maldonado R (2000) A behavioural model to reveal place preference to delta 9-tetrahydrocannabinol in mice. Psychopharmacology 147:436–438. https://doi.org/10.1007/s002130050013
Morales M, Root DH (2014) Glutamate neurons within the midbrain dopamine regions. Neuroscience 282:60–68. https://doi.org/10.1016/j.neuroscience.2014.05.032
Morales M, Margolis EB (2017) Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci 18:73–85. https://doi.org/10.1038/nrn.2016.165
Dong Y, Saal D, Thomas M, Faust R, Bonci A, Robinson T, Malenka RC (2004) Cocaine-induced potentiation of synaptic strength in dopamine neurons: Behavioral correlates in GluRA(-/-) mice. Proc Natl Acad Sci 101:14282–14287. https://doi.org/10.1073/pnas.0401553101
Sachser RM, Santana F, Crestani AP, Lunardi P, Pedraza LK, Quillfeldt JA, Hardt O, De Oliveira Alvares L (2016) Forgetting of long-term memory requires activation of NMDA receptors, L-type voltage-dependent Ca2+ channels, and calcineurin. Sci Rep 6:1–9. https://doi.org/10.1038/srep22771
Liu Y, Du S, Lv L, Lei B, Shi W, Tang Y, Wang L, Zhong Y (2016) Hippocampal activation of Rac1 regulates the forgetting of object recognition memory. Curr Biol 26:2351–2357. https://doi.org/10.1016/j.cub.2016.06.056
Heasman SJ, Ridley AJ (2008) Mammalian Rho GTPases : new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 9:690–701. https://doi.org/10.1038/nrm2476
Migues PV, Liu L, Archbold GEB, Einarsson EO, Wong J, Bonasia XK, Ko SH, Wang YT et al (2016) Blocking synaptic removal of GluA2-containing AMPA receptors prevents the natural forgetting of long-term memories. J Neurosci 36:3481–3494. https://doi.org/10.1523/JNEUROSCI.3333-15.2016
Ranaldi R, Wise RA (2001) Blockade of D1 dopamine receptors in the ventral tegmental area decreases cocaine reward: possible role for dendritically released dopamine. J Neurosci 21:5841–5846
Acknowledgments
We thank Dr. Cynthia Katche, Ana Belén de Landeta and Magdalena Pereyra for their help with the biochemistry experiments and daily work in general.
Funding
This study was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, Argentina) to JHM; nos. 2013–0335 and 2016-0034; Universidad de Buenos Aires (UBACyT, Argentina) 2014–2017 and the Argentina National Research Council (Consejo Nacional de Investigaciones Científicas y Técnicas, CONICET).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Figure S1
Histological analysis of cannulae placement. Schematic illustration of the injections sites in the intended areas. Top: VTA, hippocampus and mPFC; Bottom: NAc and BLA. Coordinates were measured from Bregma taken from the atlas of Paxinos and Watson (2005). (PNG 717 kb)
Rights and permissions
About this article
Cite this article
Castillo Díaz, F., Hernandez, M.A., Capellá, T. et al. Dopamine Neurotransmission in the Ventral Tegmental Area Promotes Active Forgetting of Cocaine-Associated Memory. Mol Neurobiol 56, 6206–6217 (2019). https://doi.org/10.1007/s12035-019-1516-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1516-3