Abstract
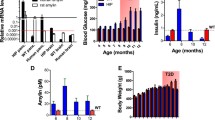
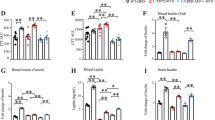
Current evidence suggests dementia and pathology in Alzheimer’s Disease (AD) are both dependent and independent of amyloid processing and can be induced by multiple ‘hits’ on vital neuronal functions. Type 2 diabetes (T2D) poses the most important risk factor for developing AD after ageing and dysfunctional IR/PI3K/Akt signalling is a major contributor in both diseases. We developed a model of T2D, coupling subdiabetogenic doses of streptozotocin (STZ) with a human junk food (HJF) diet to more closely mimic the human condition. Over 35 weeks, this induced classic signs of T2D (hyperglycemia and insulin dysfunction) and a modest, but stable deficit in spatial recognition memory, with very little long-term modification of proteins in or associated with IR/PI3K/Akt signalling in CA1 of the hippocampus. Intracerebroventricular infusion of soluble amyloid beta 42 (Aβ42) to mimic the early preclinical rise in Aβ alone induced a more severe, but short-lasting deficits in memory and deregulation of proteins. Infusion of Aβ on the T2D phenotype exacerbated and prolonged the memory deficits over approximately 4 months, and induced more severe aberrant regulation of proteins associated with autophagy, inflammation and glucose uptake from the periphery. A mild form of environmental enrichment transiently rescued memory deficits and could reverse the regulation of some, but not all protein changes. Together, these data identify mechanisms by which T2D could create a modest dysfunctional neuronal milieu via multiple and parallel inputs that permits the development of pathological events identified in AD and memory deficits when Aβ levels are transiently effective in the brain.













Similar content being viewed by others
References
Ono K (2018) Alzheimer’s disease as oligomeropathy. Neurochem Int 119:57–70. https://doi.org/10.1016/j.neuint.2017.08.010
Skaper SD (2012) Alzheimer’s disease and amyloid: culprit or coincidence? Int Rev Neurobiol 102:277–316. https://doi.org/10.1016/B978-0-12-386986-9.00011-9
Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R et al (2008) Long-term effects of Aβ 42 immunisation in Alzheimer’s disease : follow-up of a randomised, placebo-controlled phase I trial. Lancet 372(9634):216–223. https://doi.org/10.1016/S0140-6736(08)61075-2
Driscoll I, Zhou Y, An Y, Sojkova J, Davatzikos C, Kraut MA, Ye W, Ferrucci L et al (2011) Lack of association between 11C-PiB and longitudinal brain atrophy in non-demented older individuals. Neurobiol Aging 32(12):2123–2130. https://doi.org/10.1016/j.neurobiolaging.2009.12.008
Maarouf CL, Daugs ID, Kokjohn TA, Walker DG, Hunter JM, Kruchowsky JC, Woltjer R, Kaye J et al (2011) Alzheimer's disease and non-demented high pathology control nonagenarians: comparing and contrasting the biochemistry of cognitively successful aging. PLoS One 6(11):e27291. https://doi.org/10.1371/journal.pone.0027291
Atwood CS, Bowen RL (2015) A unified hypothesis of early-and late-onset Alzheimer’s disease pathogenesis. J Alzheimers Dis 47(1):33–47. https://doi.org/10.3233/JAD-143210
De Felice FG, Lourenco ML, Ferreira ST (2014) How does brain insulin resistance develop in Alzheimer’s disease? Alzheimers Dement 10(1 suppl):S26–S32. https://doi.org/10.1016/j.jalz.2013.12.004
de Wilde MC, Vellas B, Girault E, Yavuz AC, Sijben JW (2017) Lower brain and blood nutrient status in Alzheimer’s disease: results from meta-analyses. Alzheimers Dement 3(3):416–431. https://doi.org/10.1016/j.trci.2017.06.002
Erickson MA, Banks WA (2013) Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J Cereb Blood Flow Metab 33(10):1500–1513. https://doi.org/10.1038/jcbfm.2013.135
Galvan V, Hart MJ (2016) Vascular mTOR-dependent mechanisms linking the control of aging to Alzheimer’s disease. Biochim Biophys Acta 1862:992–1007. https://doi.org/10.1016/j.bbadis.2015.11.010
Gerakis Y, Hetz C (2018) Emerging roles of ER stress in the etiology and pathogenesis of Alzheimer’s disease. FEBS J 285(6):995–1011. https://doi.org/10.1111/febs.14332
Gibas KJ (2017) The starving brain: Overfed meets undernourished in the pathology of mild cognitive impairment (MCI) and Alzheimer’s disease (AD). Neurochem Int 110:57–68. https://doi.org/10.1016/j.neuint.2017.09.004
Uddin S, Stachowiak A, Al Mamun A, Tzvetkov NT, Takeda S, Atanasov AG, Bergantin LB, Abdel-Daim MM et al (2018) Autophagy and Alzheimer’s disease: from molecular mechanisms to therapeutic implications. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2018.00004
Yin F, Sancheti H, Patil I, Cadenas E (2016) Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic Biol Med 100:108–122. https://doi.org/10.1016/j.freeradbiomed.2012.04.200
Paul KC, Jerrett M, Ritz B (2018) Type 2 diabetes mellitus and Alzheimer’s disease: overlapping biologic mechanisms and environmental risk factors. Curr Environ Health Rep 5(1):1–15. https://doi.org/10.1007/s40572-018-0176-1
Gorska-Ciebiada M, Saryusz-Wolska M, Ciebiada M, Loba J (2014) Mild cognitive impairment and depressive symptoms in elderly patients with diabetes: prevalence, risk factors, and comorbidity. J Diabetes Res 2014:1–7. https://doi.org/10.1155/2014/179648
Jack CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ (2010) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9(1):119–128. https://doi.org/10.1016/S1474-4422(09)70299-6
Leitner DR, Frühbeck G, Yumuk V, Schindler K, Micic D, Woodward E, Toplak H (2017) Obesity and type 2 diabetes: two diseases with a need for combined treatment strategies - EASO can lead the way. Obes Facts 10(5):483–492. https://doi.org/10.1159/000480525
Templeman NM, Skovso S, Page MM, Lim GE, Johnson JD (2017) A causal role for hyperinsulinemia in obesity. J Endocrinol 232(3):R173–R183. https://doi.org/10.1530/JOE-16-0449
Daulatzai MA (2017) Cerebral hypoperfusion and glucose hypometabolism: key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer's disease. J Neurosci Res 95(4):943–972. https://doi.org/10.1002/jnr.23777
Vieira MNN, Lima-Filho RAS, De Felice FG (2018) Connecting Alzheimer’s disease to diabetes: underlying mechanisms and potential therapeutic targets. Neuropharmacol 136(Pt B):160–171. https://doi.org/10.1016/2017.11.014
Verdile G, Fuller SJ, Martins RN (2015) The role of type 2 diabetes in neurodegeneration. Neurobiol Dis 84:22–38. https://doi.org/10.1016/j.nbd.2015.04.008
Pruzin JJ, Nelson PT, Abner EL, Arvanitakis Z (2018) Relationship of type 2 diabetes to human brain pathology. Neuropathol Appl Neurobiol 44(4):347–362. https://doi.org/10.1111/nan.12476
Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW (2004) Incipient Alzheimer’s disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci 101(7):2173–2178. https://doi.org/10.1073/pnas.0308512100
Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR et al (2005) Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—is this type 3 diabetes? J Alzheimers Dis 7(1):63–80. https://doi.org/10.3233/JAD-2005-7107
Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, O'Connor R, O'Neill C (2005) Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J Neurochem 93(1):105–117. https://doi.org/10.1111/j.1471-4159.2004.02949.x
Busquets O, Ettcheto M, Pallàs M, Beas-Zarate C, Verdaguer E, Auladell C, Folch J, Camins A (2017) Long-term exposition to a high fat diet favors the appearance of β-amyloid depositions in the brain of C57BL/6J mice. A potential model of sporadic Alzheimer’s disease. Mech Ageing Dev 162:38–45. https://doi.org/10.1016/j.mad.2016.11.002
Salas IH, Weerasekera A, Ahmed T, Callaerts-Vegh Z, Himmelreich U, D’Hooge R, Balschun D, Saido TC et al (2018) High fat diet treatment impairs hippocampal long term potentiation without alterations of the core neuropathological features of Alzheimer’s disease. Neurobiol Dis 113:82–96. https://doi.org/10.1016/j.nbd.2018.02.001
Carvalho C, Cardoso S, Correia SC, Santos RX, Santos MS, Baldeiras I, Oliveira CR, Moreira PI (2012) Metabolic alterations induced by sucrose intake and Alzheimer’s disease promotes similar brain mitochondrial abnormalities. Diabetes 61(5):1234–1242. https://doi.org/10.1016/j.nbd.2018.02.001
Hiltunen M, Khandelwal VKM, Yaluri N, Tiilikainen T, Tusa M, Koivisto H, Krzisch M, Vepsäläinen S et al (2012) Contribution of genetic and dietary insulin resistance to Alzheimer phenotype in APP/PS1 transgenic mice. J Cell Mol Med 16(6):1206–1222. https://doi.org/10.1111/j.1582-4934.2011.01384.x
Ramos-Rodriguez JJ, Spires-Jones T, Pooler AM, Lechuga-Sancho AM, Bacskai BJ, Garcia-Alloza M (2017) Progressive neuronal pathology and synaptic loss induced by prediabetes and type 2 diabetes in a mouse model of Alzheimer’s disease. Mol Neurobiol 54(5):3428–3438. https://doi.org/10.1007/s12035-016-9921-3
Alagiakrishnan K, Sankaralingam S, Ghosh M, Mereu L, Senior P (2013) Antidiabetic drugs and their potential role in treating mild cognitive impairment and Alzheimer’s disease. Discov Med 16(90):277–286
Holscher C (2018) Novel dual GLP-1/GIP receptor agonists show neuroprotective effects in Alzheimer’s and Parkinson’s diease models. Neuropharmacol 136(PtB):251–259. https://doi.org/10.1016/j.neuropham.2018.01.040
Grieb P (2016) Intracerebroventricular Streptozotocin injections as a model of Alzheimer’s disease: in search of a relevant mechanism. Mol Neurobiol 53:1741–1752. https://doi.org/10.1007/s12035-015-9132-3
Kamat PK, Kalani A, Rai S, Tota SK, Kumar A, Ahmad AS (2016) Streptozotocin Intracerebroventricular-induced neurotoxicity and brain insulin resistance: a therapeutic intervention for treatment of sporadic Alzheimer’s disease (sAD)-like pathology. Mol Neurobiol 53(7):4548–4562. https://doi.org/10.1007/s12035-015-9384-y
Chami B, Steel AJ, De La Monte SM, Sutherland GT (2016) The rise and fall of insulin signaling in Alzheimer’s disease. Metab Brain Dis 31(3):497–515. https://doi.org/10.1007/s11011-016-9806-1
Zhang M, Lv X-Y, Xu Z-G, Chen L (2008) The characterization of highfat diet and multiple low-dow Streptozotocin induced type 2 diabetes rat model. Exp Diabet Res 704045. https://doi.org/10.1155/2008/704045.
Skovso S (2014) Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J Diabetes Investig 5(4):349–358. https://doi.org/10.1111/jdi.12235
Ohline SM, Abraham WC (2018) Environmental enrichment effects on synaptic and cellular physiology of hippocampal neurons. Neuropharmacol. https://doi.org/10.1016/j.neuropharm.2018.04.007
Costa DA, Cracchiolo JR, Bachstetter AD, Hughes TF, Bales KR, Paul SM, Mervis RF, Arendash GW et al (2007) Enrichment improves cognition in AD mice by amyloid-related and unrelated mechanisms. Neurobiol Aging 28(6):831–844. https://doi.org/10.1016/j.neurobiolaging.2006.04.009
Bozso Z, Penke B, Simon D, Laczkó I, Juhász G, Szegedi V, Kasza A, Soós K et al (2010) Controlled in situ preparation of a beta(1-42) oligomers from the isopeptide “iso-A beta(1-42)”, physicochemical and biological characterization. Peptides 31(2):248–256. https://doi.org/10.1016/j.peptides.2009.12.001
Bruel-Jungerman E, Laroche S, Rampon C (2005) New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci 21(2):513–521. https://doi.org/10.1111/j.1460-9568.2005.03875.x
Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S (2000) The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci 20(12):4563–4572. https://doi.org/10.1523/JNEUROSCI.20-12-04563.2000
Kayed R, Head E, Sarsoza F, Saing T, Cotman CW, Necula M, Margol L, Wu J et al (2007) Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener 26:2–18. https://doi.org/10.1186/1750-1326-2-18
Holder MK, Chassaing B (2018) Impact of food additives on the gut-brain axis. Physiol Behav 192:173–176. https://doi.org/10.1016/j.physbeh.2018.02.025
Bray GA (1977) The Zucker-fatty rat: a review. Fed Proc 36(2):148–153
Harishankar N, Vajreswari A, Giridharan NV (2011) WNIN/GR-Ob an insulin-resistant obese rat model from inbred WNIN strain. Indian J Med Res 134:320–329
Shafat A, Murray B, Rumsey D (2009) Energy density in cafeteria diet induced hyperphagia in the rat. Appetite 52(1):34–38. https://doi.org/10.1016/j.appet.2008.07.004
Levin BE, Dunn-Meynell AA (2002) Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Phys Regul Integr Comp Phys 283(5):R941–R948. https://doi.org/10.1152/ajpregu.00245.2002
Woods SC, Seeley RJ, Rushing PA, D’Alessio D, Tso P (2003) A controlled high-fat diet induces an obese syndrome in rats. J Nutr 133(4):1081–1087. https://doi.org/10.1093/jn/133.4.1081
Leahy JL (2005) Pathogenesis of type 2 diabetes mellitus. Arch Med Res 36(3):197–209. https://doi.org/10.1016/j.arcmed.2005.01.003
Alejandro EU, Gregg B, Blandino-Rosano M, Cras-Méneur C, Bernal-Mizrachi E (2014) Natural history of beta-cell adaptation and failure in type 2 diabetes. Mol Asp Med 42:19–41. https://doi.org/10.1016/j.mam.2014.12.002
Wong RS, Cechetto DF, Whitehead SN (2016) Assessing the effects of acute amyloid β oligomer exposure in the rat. Int J Mol Sci 17(9):1390–1403. https://doi.org/10.3390/ijms17091390
Ma YQ, Wu DK, Liu JK (2013) mTOR and tau phosphorylated proteins in the hippocampal tissue of rats with type 2 diabetes and Alzheimer’s disease. Mol Med Rep 7(2):623–627. https://doi.org/10.3892/mmr.2012.1186
Knight EM, Martins IVA, Gümüsgöz S, Allan SM, Lawrence CB (2014) High-fat diet-induced memory impairment in triple-transgenic Alzheimer’s disease (3xTgAD) mice is independent of changes in amyloid and tau pathology. Neurobiol Aging 35(8):1821–1832. https://doi.org/10.1016/j.neurobiolaging.2014.02.010
Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, Peng Y, Cambareri G et al (2004) Diet induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer’s disease. FASEB J 18(7):902–924. https://doi.org/10.1096/fj.03-0978fje
Cao D, Lu H, Lewis TL, Li L (2007) Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J Biol Chem 282(50):36275–36282. https://doi.org/10.1074/jbc.M703561200
Vandal M, White PJ, Tremblay C, St-Amour I, Chevrier G, Emond V, Lefrancois D, Virgili J et al (2014) Insulin reverses the high-fat diet-induced brain Aβ and improves memory in an animal model of Alzheimer’s disease. Diabetes 63(12):4291–4301. https://doi.org/10.2337/db14-0375
Fitz NF, Cronican A, Pham T, Fogg A, Fauq AH, Chapman R, Lefterov I, Koldamova R (2010) Liver X receptor agonist treatment ameliorates amyloid pathology and memory deficits caused by high-fat diet in APP23 mice. J Neurosci 30(20):6862–6872. https://doi.org/10.1523/JNEUROSCI.1051-10.2010
Herculano B, Tamura M, Ohba A, Shimatani M, Kutsuna N, Hisatsune T (2013) β-Alanyl-L-histidine rescues cognitive deficits caused by feeding a high fat diet in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis 33(4):983–997. https://doi.org/10.3233/JAD-2012-121324
Maesako M, Uemura K, Kubota M, Kuzuya A, Sasaki K, Asada M, Watanabe K, Hayashida N et al (2012) Environmental enrichment ameliorated high-fat diet-induced Aβ deposition and memory deficit in APP transgenic mice. Neurobiol Aging 33(5):1011.e11–1011.e23. https://doi.org/10.1016/j.neurobiolaging.2011.10.028
Herrup K (2010) Reimagining Alzheimer’s disease—an age-based hypothesis. J Neurosci 30(50):16755–16762. https://doi.org/10.1523/JNEUROSCI.4521-10.2010
Herrup K, Carrillo MC, Schenk D, Cacace A, DeSanti S, Fremeau RF, Bhat R, Glicksman M et al (2013) Beyond amyloid: getting real about nonamyloid targets in Alzheimer’s disease. Alzheimers Dement 9(4):452–458. https://doi.org/10.1016/j.jalz.2013.01.017
Redolat R, Mesa-Gresa P (2012) Potential benefits and limitations of enriched environments and cognitive activity on age-related behavioural decline. Curr Top Behav Neurosci 10:293–316. https://doi.org/10.1007/7854_2011_134
Prado Lima MG, Schimidt HL, Garcia A, Daré LR, Carpes FP, Izquierdo I, Mello-Carpes PB (2018) Environmental enrichment and expercise are better than social enrichment to reduce memory deficits in amyloid beta neurotoxicity. Proc Natl Acad Sci 115(10):E2403–E2409. https://doi.org/10.1073/pnas.1718435115
La Joie R, Perrotin A, de la Sayette V, Egret S, Doeuvre L; Belliard S, Eustache F, Desgranges B, Chételat G (2013) Hippocampal subfield volumetry in mild cognitive impairment, Alzheimer’s disease and semantic dementia. Neuroimage Clin 3:155–162. https://doi.org/10.1016/j.nicl.2013.08.007
Counts SE, Alldred MJ, Che S, Ginsberg SD, Mufson EJ (2013) Synaptic gene dysregulation within hippocampal CA1 pyramidal neurons in mild cognitive impairment. Neuropharmacology 79:172–179. https://doi.org/10.1016/j.neuropharm.2013.10.018
Masurkar AV (2018) Towards a circuit-level understanding of hippocampal CA1 dysfunction in Alzheimer’s disease across anatomical axes. J Alzheimers Dis Parkinsonism 8(1).
Jimenez S, Torres M, Vizuete M, Sanchez-Varo R, Sanchez-Mejias E, Trujillo-Estrada L, Carmona-Cuenca I, Caballero C et al (2011) Age-dependent accumulation of soluble amyloid beta (Aβ) oligomers reverses the neuroprotective effect of soluble amyloid precursor protein-α (sAPPα) by modulating phosphatidylinositol 3-kinase (PI3K)/Akt-GSK-3β pathway in Alzheimer mouse model. J Biol Chem 286:18414–18425
Qutub AA, Hunt CA (2005) Glucose transport to the brain: a systems model. Brain Res Brain Res Rev 49(3):595–617. https://doi.org/10.1016/j.brainresrev.2005.03.002
Martin L, Latypova X, Wilson CM, Magnaudeix A, Perrin A-L, Yardin C, Terro F (2013) Tau protein kinases: involvement in Alzheimer’s Disease. Ageing Res Rev 12(1):289–309. https://doi.org/10.1016/j.arr.2012.06.003
Jaeger PA, Wyss-Coray T (2010) Beclin 1 complex in autophagy and Alzheimer disease. Arch Neurol 67(10):1181–1184. https://doi.org/10.1001/archneurol.2010.258
Caccamo A, Magr A, Medina DX, Silva AJ, Wisely EV, Manuel FL, Oddo S (2013) mTOR regulates tau phosphorylation and degradation: implications for Alzheimer’s disease and other tauopathies. Aging Cell 12(3):370–380. https://doi.org/10.1111/acel.12057
Farr SA, Ripley JL, Sultana R, Zhang Z, Niehoff ML, Platt TL, Murphy MP, Morely JE et al (2014) Antisense oligonucleotide against GSK-3β in brain of SAMP8 mice improves learning and memory and decreases oxidative stress: Involvement of transcription factor Nrf2 and implications for Alzheimer disease. Free Radic Biol Med 67:387–395. https://doi.org/10.1016/j.freeradbiomed.2013.11.014
Tramutola A, Triplett JC, Di Domenico F, Niedowicz DM, Murphy MP, Coccia R, Perluigi M, Butterfield DA (2015) Alteration of mTOR signalling occurs early in the progression of Alzheimer disease (AD): analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD. J Neurochem 133(5):739–749. https://doi.org/10.1111/jnc.13037
Liu Y, Grundke-Iqbal FLI, Gong KI, Gong C-X (2011) Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J Pathol 225(1):54–62. https://doi.org/10.1002/path.2912
Maqbool M, Mobashir M, Hoda N (2016) Pivital role of glycogen synthase kinase-3: a therapeutic target for Alzheimer’s disease. Eur J Med Chem 107:63–81. https://doi.org/10.1016/j.emech.2015.10.018
Lee YS, Chow WNV, Lau K-F (2017) Phosphorylation of FE65 at threonine 579 by GSK3β stimulates amyloid precursor protein processing. Sci Rep 7(1):12456. https://doi.org/10.1038/s41598-017-12334-2
Qiu WQ, Folstein MF (2006) Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer's disease: review and hypothesis. Neurobiol Aging 27(2):190–198
Vepsäläinen S, Hiltunen M, Helisalmi S, Wang J, van Groen T, Tanila H, Soininen H (2008) Increased expression of Abeta degrading enzyme IDE in the cortex of transgenic mice with Alzheimer’s disease-like neuropathology. Neurosci Lett 438(2):216–220. https://doi.org/10.1016/j.neulet.2008.04.025
Hooper C, Killick R, Lovestone S (2008) The GSK3 hypothesis of Alzheimer’s disease. J Neurochem 104(6):1433–1439. https://doi.org/10.1111/j.1471-4159.2007.05194.x
Huang K, Fingar DC (2014) Growing knowledge of the mTOR signaling network. Semin Cell Dev Biol 36:79–90. https://doi.org/10.1016/j.semcdb.2014.09.011
Sun YX, Ji X, Mao X, Xie L, Jia J, Galvan V, Greenberg DA, Jin K (2014) Differential activation of mTOR complex 1 signaling in human brain with mild to severe Alzheimer’s disease. J Alzheimers Dis 38(2):437–444. https://doi.org/10.3233/JAD-131124
Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredensen D, Richardson A, Strong R et al (2010) Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-β levels in a mouse model of Alzheimer’s disease. PLoS One 5(4):e9979. https://doi.org/10.1371/journal.pone.0009979
Caccamo A, Belfiore R, Oddo S (2018) Genetically reducing mTOR signaling rescues central insulin dysregulation in a mouse model of Alzheimer's disease. Neurobiol Aging 68:59–67. https://doi.org/10.1016/j.neurobiolaging.2018.03.032
Caccamo A, De Pinto V, Messina A, Branca C, Oddo S (2014) Genetic reduction of mammalian target of rapamycin ameliorates Alzheimer’s disease-like cognitive and pathological deficits by restoring hippocampal gene expression signature. J Neurosci 34(23):7988–7998. https://doi.org/10.1523/JNEUROSCI.0777-14.2014
Holz MK, Blenis J (2005) Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J Biol Chem 280(28):26089–26093. https://doi.org/10.1074/jbc.M504045200
François A, Rioux-Bilan A, Quellard N, Fernandez B, Janet T, Chassaing D, Paccalin M, Terro F et al (2014) Longitudinal follow-up of autophagy and inflammation in brain of APPswePS1dE9 transgenic mice. J Neuroinflammation 11:139. https://doi.org/10.1186/s12974-014-0139
Zhang Y, Sowers JR, Ren J (2018) Targeting autophagy in obesity: from pathophysiology to management. Nat Rev Endocrinol 14(6):356–376. https://doi.org/10.1038/s41574-018-0009-1
Kim J, Lim YM, Lee MS (2018) The role of autophagy in systemic metabolism and human-type diabetes. Mol Cells 41(1):11–17. https://doi.org/10.14348/molcells.2018.2228
Skaper SD, Facci L, Zusso M, Giusti P (2018) An inflammation-centric view of neurological disease: beyond the neuron. Front Cell Neurosci 12:72. https://doi.org/10.3389/fncel.2018.00072
Pompl PN, Yemul S, Xiang Z, Ho L, Haroutunian V, Purohit D, Mohs R, Pasinetti GM (2003) Caspase gene expression in the brain as a function of the clinical progression of Alzheimer disease. Arch Neurol 60(3):369–376. https://doi.org/10.1001/archneur.60.3.369
Obulesu M, Lakshmi MJ (2014) Apoptosis in Alzheimer’s disease: an understanding of the physiology, pathology and therapeutic avenues. Neurochem Res 39(12):2301–2312. https://doi.org/10.1007/s11064-014-1454-4
Maiese K (2008) Triple play: promoting neurovascular longevity with nicotinamide, wnt and erythropoietin in diabetes mellitus. Biomed Pharmacother 62(4):218–232. https://doi.org/10.1016/j.biopha.2008.01.009
van Dijk G, van Heijningen S, Reijne AC, Nyakas C, van der Zee EA, Eisel UL (2015) Integrative neurobiology of metabolic diseases, neuroinflammation, and neurodegeneration. Front Neurosci 9:173. https://doi.org/10.3389/fnins.2015.00173
Ljubicic S, Polak K, Fu A, Wiwczar J, Szlyk B, Chang Y, Alvez-Perez JC, Bird GH et al (2015) Phospho-BAD BH3 mimicry protects β cell and restores functional β cell mass in diabetes. Cell Rep 10(4):497–504. https://doi.org/10.1016/j.celrep.2014.12.056
van Greevenbroek MMJ, Schalkwijk CG, Stehouwer CDA (2013) Obesity-associated low-grade inflammation in type 2 diabetes mellitus: causes and consequences. Neth J Med 71(4):174–187
Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME (1997) Akt phosphorylation of BAD couples signals to the cell-intrinsic death machinery. Cell 91:231–241
Leong ML, Maiyar AC, Kim B, O'Keeffe BA, Firestone GL (2003) Expression of the serum- and glucocorticoid-inducible protein kinase, Sgk, is a cell survival response to multiple types of environmental stress stimuli in mammary epithelial cells. J Biol Chem 278(8):5871–5882. https://doi.org/10.1074/jbc.M211649200
Shi C, Viccaro K, Lee H-G, Sha K (2016) Cdk5-Foxo3 axis: initially neuroprotective, eventually neurodegenerative in Alzheimer’s disease models. J Cell Sci 129:1815–1830. https://doi.org/10.1242/jcs.185009
Qin W, Zhao W, Ho L, Wang J, Walsh K, Gandy S, Pasinetti GM (2009) Regulation of forkhead transcription factor FoxO3a contributes to calorie restriction-induced prevention of Alzheimer's disease-type amyloid neuropathology and spatial memory deterioration. Ann N Y Acad Sci 1147:335–347. https://doi.org/10.1196/annals.1427.024
Qin W, Zhao W, Ho L, Wang J, Walsh K, Gandy S, Pasinetti GM (2008) Regulation of forkhead transcription factor FoxO3a contributes to calorie restriction-induced prevention of Alzheimer’s disease-type amyloid neuropathology and spatial memory deterioration. Ann N Y Acad Sci 1147:335–347. https://doi.org/10.1196/annals.1427.024
Nuzzo D, Picone P, Baldassano S, Caruana L, Messina E, Marino Gammazza A, Cappello F, Mulè F et al (2015) Insulin resistance as common molecular denominator linking obesity to Alzheimer’s disease. Curr Alzheimer Res 12(8):723–35.94
Martins R, Lithgow GJ, Link W (2016) Long live FOXO: unravelling the role of FOXO proteins in ageing and longevity. Aging Cell 15(2):196–207. https://doi.org/10.1111/acel.12427
Shah K, DeSilva S, Abbruscato T (2012) The role of glucose transporters in brain disease: diabetes and Alzheimer’s disease. Int J Mol Sci 13(10):12629–12655. https://doi.org/10.3390/ijms131012629
Meneilly GS, Tessier DM (2016) Diabetes, dementia and hypoglycemia. Can J Diabetes 40(1):73–76. https://doi.org/10.1016/j.jcjd.2015.09.006
Sato N, Morishita R (2013) Roles of vascular and metabolic components in cognitive dysfunction of Alzheimer’s disease: short-and long-term modification by non-genetic risk factors. Front Aging Neurosci 5(64). https://doi.org/10.3389/fnagi.2013.00064
Montagne A, Zhao Z, Zlokovic BV (2017) Alzheimer’s disease: a matter of blood-brain barrier dysfunction? J Exp Med 214(11):3151–3169. https://doi.org/10.1084/jem.20171406
Niwa K, Younkin L, Ebeling C, Turner SK, Westaway D, Younkin S, Hsiao-Ashe K, Carlson GA et al (2000) Aβ-40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci USA 97(17):9735–9740. https://doi.org/10.1073/pnas.97.17.9735
Ly PTT, Wu Y, Zou H, Wang R, Zhou W, Kinoshita A, Zhang M, Yang Y et al (2013) Inhibition of GSK3β-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J Clin Invest 123(1):224–235. https://doi.org/10.1172/JCI64516
Decourt B, Lahiri DK, Sabbagh MN (2017) Targeting tumor necrosis factor alpha for Alzheimer’s disease. Curr Alzheimer Res 14(4):412–425. https://doi.org/10.2174/1567205013666160930110551
Ahmad W, Ijaz B, Shabbiri K, Ahmed F, Rehman S (2017) Oxidative toxicity in diabetes and Alzheimer’s disease: mechanisms behind ROS/RNS generation. J Biomed Sci 24(1):76. https://doi.org/10.1186/s12929-017-0379-z
Salminen A, Kaarniranta K, Haapasalo A, Soininen H, Hiltunen M (2011) AMP-activated protein kinase: a potential player in Alzheimer’s disease. J Neurochem 118(4):460–474. https://doi.org/10.1111/j.1471-4159.2011.07331.x
Cai Z, Yan LJ, Li K, Quazi SH, Zhao B (2012) Roles of AMP-activated protein kinase in Alzheimer’s disease. Neuromol Med 14(1):1–14. https://doi.org/10.1007/s12017-012-8173-2
Metaxakis A, Ploumi C, Tavernarakis N (2018) Autophagy in age-associated neurodegeneration. Cells 7(5):E37. https://doi.org/10.1186/s12929-017-0379-z
Acknowledgements
This work was supported by the Centre National de la Recherche Scientifique (CNRS, France); LECMA Vaincre Alzheimer, France (12702) to SD; the Hungarian Brain Research Programme 2.0 (2017-1.2.1-NKP-2017-00002) and Economy Development and Innovation Operative Programme (GINOP 2.3.2_15_2016_00038) to LF and ZB. KN was supported by LECMA funding and MS by the China Scholarship Council.
Author information
Authors and Affiliations
Contributions
SD conceived and designed the research. MS, KN, NS and IY conducted the experiments. SD, BD, MS, KN and IY analysed data. IY and BD conducted all immunohistochemistry; LF and ZB produced and tested Aβ1-42 peptide. SD drafted the paper with contributions from MS and SL, and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 29 kb)
Rights and permissions
About this article
Cite this article
Su, M., Naderi, K., Samson, N. et al. Mechanisms Associated with Type 2 Diabetes as a Risk Factor for Alzheimer-Related Pathology. Mol Neurobiol 56, 5815–5834 (2019). https://doi.org/10.1007/s12035-019-1475-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1475-8