Abstract
The majority of mutations in rhodopsin (RHO) cause misfolding of the protein and has been linked to degeneration of photoreceptor cells in the retina. A lot of attention has been set on targeting ER stress for the development of new therapies for inherited retinal degeneration caused by mutations in the RHO gene. Nevertheless, the cell death pathway activated by RHO misfolded protein is still debated. In this study, we analyzed the retina of the knock-in mouse expressing the P23H misfolded mutant RHO. We found persistent unfolded protein response (UPR) during degeneration. Interestingly, long-term stimulation of the PERK branch of ER stress had a protective effect by phosphorylating nuclear factor erythroid 2–related factor 2 (NRF2) transcription factor, associated with antioxidant responses. Otherwise, we provide evidence that increased intracellular calcium and activation of calpains strongly correlated with rod photoreceptor cell death. By blocking calpain activity, we significantly decreased the activation of caspase-7 and apoptosis-inducing factor (AIF), two cell death effectors, and cell demise, and effectively protected the retina from degeneration caused by the P23H dominant mutation in RHO.




Similar content being viewed by others
References
Hartong DT, Berson EL, Dryja TP (2006) Retinitis pigmentosa. Lancet. 368:1795–1809
Dryja TP, McGee TL, Reichel E, Hahn LB, Cowley GS, Yandell DW et al (1990) A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 343:364–366
Behnen P, Felline A, Comitato A, Di Salvo MT, Raimondi F, Gulati S et al (2018) A small chaperone improves folding and routing of rhodopsin mutants linked to inherited blindness. iScience 4:1–19
Gorbatyuk MS, Knox T, LaVail MM, Gorbatyuk OS, Noorwez SM, Hauswirth WW et al (2010) Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc Natl Acad Sci U S A 107:5961–5966
Price BA, Sandoval IM, Chan F, Simons DL, Wu SM, Wensel TG, Wilson JH (2011) Mislocalization and degradation of human P23H-rhodopsin-GFP in a knockin mouse model of retinitis pigmentosa. Invest Ophthalmol Vis Sci 52:9728–9736
Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, LaVail MM et al (2007) IRE1 signaling affects cell fate during the unfolded protein response. Science. 318:944–949
McCullough KD, Martindale JL, Klotz L-O, Aw T-Y, Holbrook NJ (2001) Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21:1249–1259
Kunte MM, Choudhury S, Manheim JF, Shinde VM, Miura M, Chiodo VA, Hauswirth WW, Gorbatyuk OS et al (2012) ER stress is involved in T17M rhodopsin-induced retinal degeneration. Invest Ophthalmol Vis Sci 53:3792–3800
Kroeger H, Messah C, Ahern K, Gee J, Joseph V, Matthes MT, Yasumura D, Gorbatyuk MS et al (2012) Induction of endoplasmic reticulum stress genes, BiP and chop, in genetic and environmental models of retinal degeneration. Invest Ophthalmol Vis Sci 53:7590–7599
Ohgane K, Dodo K, Hashimoto Y (2010) Retinobenzaldehydes as proper-trafficking inducers of folding-defective P23H rhodopsin mutant responsible for retinitis pigmentosa. Bioorg Med Chem 18:7022–7028
Athanasiou D, Bevilacqua D, Aguila M, McCulley C, Kanuga N, Iwawaki T, Paul Chapple J, Cheetham ME (2014) The co-chaperone and reductase ERdj5 facilitates rod opsin biogenesis and quality control. Hum Mol Genet 23:6594–6606
Chen Y, Chen Y, Jastrzebska B, Golczak M, Gulati S, Tang H, Seibel W, Li X et al (2018) A novel small molecule chaperone of rod opsin and its potential therapy for retinal degeneration. Nat Commun 9:1976
Mendes HF, Zaccarini R, Cheetham ME (2010) Pharmacological manipulation of rhodopsin retinitis pigmentosa. Adv Exp Med Biol 664:317–323
Comitato A, Di Salvo MT, Turchiano G, Montanari M, Sakami S, Palczewski K et al (2016) Dominant and recessive mutations in rhodopsin activate different cell death pathways. Hum Mol Genet 25:2801–2812
Sakami S, Maeda T, Bereta G, Okano K, Golczak M, Sumaroka A, Roman AJ, Cideciyan AV et al (2011) Probing mechanisms of photoreceptor degeneration in a new mouse model of the common form of autosomal dominant retinitis pigmentosa due to P23H opsin mutations. J Biol Chem 286:10551–10567
Sakami S, Kolesnikov AV, Kefalov VJ, Palczewski K (2014) P23H opsin knock-in mice reveal a novel step in retinal rod disc morphogenesis. Hum Mol Genet 23:1723–1741
Chiang W-C, Kroeger H, Sakami S, Messah C, Yasumura D, Matthes MT, Coppinger JA, Palczewski K et al (2015) Robust endoplasmic reticulum-associated degradation of rhodopsin precedes retinal degeneration. Mol Neurobiol 52:679–695
Botta S, Marrocco E, de Prisco N, Curion F, Renda M, Sofia M, Lupo M, Carissimo A et al (2016) Rhodopsin targeted transcriptional silencing by DNA-binding. Elife. 5:e12242
Griciuc A, Aron L, Ueffing M (2011) ER stress in retinal degeneration: a target for rational therapy? Trends Mol Med 17:442–451
Ozaki T, Ishiguro S, Hirano S, Baba A, Yamashita T, Tomita H, Nakazawa M (2013) Inhibitory peptide of mitochondrial μ-calpain protects against photoreceptor degeneration in rhodopsin transgenic S334ter and P23H rats. PLoS One 8:e71650
Latella MC, Di Salvo MT, Cocchiarella F, Benati D, Grisendi G, Comitato A et al (2016) In vivo editing of the human mutant rhodopsin gene by electroporation of plasmid-based CRISPR/Cas9 in the mouse retina. Mol Ther Nucleic Acids 5:e389
Mussolino C, Sanges D, Marrocco E, Bonetti C, Di Vicino U, Marigo V et al (2011) Zinc-finger-based transcriptional repression of rhodopsin in a model of dominant retinitis pigmentosa. EMBO Mol Med 3:118–128
Comitato A, Subramanian P, Turchiano G, Montanari M, Becerra SP, Marigo V (2018) Pigment epithelium-derived factor hinders photoreceptor cell death by reducing intracellular calcium in the degenerating retina. Cell Death Dis 9:560
Comitato A, Sanges D, Rossi A, Humphries MM, Marigo V (2014) Activation of Bax in three models of retinitis pigmentosa. Invest Ophthalmol Vis Sci 55:3555–3562
Doonan F, Donovan M, Cotter TG (2005) Activation of multiple pathways during photoreceptor apoptosis in the rd mouse. Invest Ophthalmol Vis Sci 46:3530–3538
Sanges D, Comitato A, Tammaro R, Marigo V (2006) Apoptosis in retinal degeneration involves cross-talk between apoptosis-inducing factor (AIF) and caspase-12 and is blocked by calpain inhibitors. Proc Natl Acad Sci U S A 103:17366–17371
Arango-Gonzalez B, Trifunović D, Sahaboglu A, Kranz K, Michalakis S, Farinelli P, Koch S, Koch F et al (2014) Identification of a common non-apoptotic cell death mechanism in hereditary retinal degeneration. PLoS One 9:e112142
Paquet-Durand F, Azadi S, Hauck SM, Ueffing M, van Veen T, Ekström P (2006) Calpain is activated in degenerating photoreceptors in the rd1 mouse. J Neurochem 96:802–814
Pike BR, Flint J, Dave JR, Lu X-CM, Wang KKK, Tortella FC, Hayes RL (2004) Accumulation of calpain and caspase-3 proteolytic fragments of brain-derived αII-spectrin in cerebral spinal fluid after middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab 24:98–106
Arnoult D, Gaume B, Karbowski M, Sharpe JC, Cecconi F, Youle RJ (2003) Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J 22:4385–4399
Polster BM, Basanez G, Etxebarria A, Hardwick JM, Nicholls DG (2005) Calpain I induces cleavage and release of apoptosis-inducing factor from isolated mitochondria. J Biol Chem 280:6447–6454
Frederick JM, Krasnoperova NV, Hoffmann K, Church-Kopish J, Ruther K, Howes K et al (2001) Mutant rhodopsin transgene expression on a null background. Invest Ophthalmol Vis Sci 42:826–833
Griciuc A, Aron L, Piccoli G, Ueffing M (1803) Clearance of rhodopsin(P23H) aggregates requires the ERAD effector VCP. Biochim Biophys Acta 2010:424–434
Kroeger H, Chiang WC, Lin JH (2012) Endoplasmic reticulum-associated degradation (ERAD) of misfolded glycoproteins and mutant P23H rhodopsin in photoreceptor cells. Adv Exp Med Biol 723:559–565
Choudhury S, Bhootada Y, Gorbatyuk O, Gorbatyuk M (2013) Caspase-7 ablation modulates UPR, reprograms TRAF2-JNK apoptosis and protects T17M rhodopsin mice from severe retinal degeneration. Cell Death Dis 4:e528–e528
Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA (2003) Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol 23:7198–7209
Cullinan SB, Diehl JA (2004) PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem 279:20108–20117
Athanasiou D, Aguila M, Bellingham J, Kanuga N, Adamson P, Cheetham ME (2017) The role of the ER stress-response protein PERK in rhodopsin retinitis pigmentosa. Hum Mol Genet 26:4896–4905
Gafni J, Cong X, Chen SF, Gibson BW, Ellerby LM (2009) Calpain-1 cleaves and activates caspase-7. J Biol Chem 284:25441–25449
Athanasiou D, Aguila M, Bellingham J, Li W, McCulley C, Reeves PJ, Cheetham ME (2018) The molecular and cellular basis of rhodopsin retinitis pigmentosa reveals potential strategies for therapy. Prog Retin Eye Res 62:1–23
Briscoe AD, Gaur C, Kumar S (2004) The spectrum of human rhodopsin disease mutations through the lens of interspecific variation. Gene. 332:107–118
Krebs MP, Holden DC, Joshi P, Clark CL III, Lee AH, Kaushal S (2010) Molecular mechanisms of rhodopsin retinitis pigmentosa and the efficacy of pharmacological rescue. J Mol Biol 395:1063–1078
Hargrave PA (2001) Rhodopsin structure, function, and topography the Friedenwald lecture. Invest Ophthalmol Vis Sci 42:3–9
Parfitt DA, Aguila M, McCulley CH, Bevilacqua D, Mendes HF, Athanasiou D et al (2014) The heat-shock response co-inducer arimoclomol protects against retinal degeneration in rhodopsin retinitis pigmentosa. Cell Death Dis 5:e1236
Viringipurampeer IA, Metcalfe AL, Bashar AE, Sivak O, Yanai A, Mohammadi Z, Moritz OL, Gregory-Evans CY et al (2016) NLRP3 inflammasome activation drives bystander cone photoreceptor cell death in a P23H rhodopsin model of retinal degeneration. Hum Mol Genet 25:1501–1516
LaVail MM (1973) Kinetics of rod outer segment renewal in the developing mouse retina. J Cell Biol 58:650–661
Luo D-G, Yau K-W (2005) Rod sensitivity of neonatal mouse and rat. J Gen Physiol 126:263–269
Chen Y, Jastrzebska B, Cao P, Zhang J, Wang B, Sun W, Yuan Y, Feng Z et al (2014) Inherent instability of the retinitis pigmentosa P23H mutant opsin. J Biol Chem 289:9288–9303
Pajares M, Cuadrado A, Rojo AI (2017) Modulation of proteostasis by transcription factor NRF2 and impact in neurodegenerative diseases. Redox Biol 11:543–553
Wang J, Saul A, Roon P, Smith SB (2016) Activation of the molecular chaperone, sigma 1 receptor, preserves cone function in a murine model of inherited retinal degeneration. Proc Natl Acad Sci U S A 113:E3764–E3772
Inoue Y, Shimazawa M, Noda Y, Nagano R, Otsuka T, Kuse Y, Nakano Y, Tsuruma K et al (2017) RS9, a novel Nrf2 activator, attenuates light-induced death of cells of photoreceptor cells and Müller glia cells. J Neurochem 141:750–765
Ildefonso CJ, Jaime H, Brown EE, Iwata RL, Ahmed CM, Massengill MT, Biswal MR, Boye SE et al (2016) Targeting the Nrf2 signaling pathway in the retina with a gene-delivered secretable and cell-penetrating peptide. Invest Ophthalmol Vis Sci 57:372–386
Byrne AM, Ruiz-Lopez AM, Roche SL, Moloney JN, Wyse-Jackson AC, Cotter TG et al (2016) The synthetic progestin norgestrel modulates Nrf2 signaling and acts as an antioxidant in a model of retinal degeneration. Redox Biol 10:128–139
Nakagami Y, Hatano E, Inoue T, Yoshida K, Kondo M, Terasaki H (2016) Cytoprotective effects of a novel Nrf2 activator, RS9, in rhodopsin Pro347Leu rabbits. Curr Eye Res 41:1123–1126
Chiang W-C, Hiramatsu N, Messah C, Kroeger H, Lin JH (2012) Selective activation of ATF6 and PERK endoplasmic reticulum stress signaling pathways prevent mutant rhodopsin accumulation. Invest Ophthalmol Vis Sci 53:7159–7166
Hammadi M, Oulidi A, Gackière F, Katsogiannou M, Slomianny C, Roudbaraki M, Dewailly E, Delcourt P et al (2013) Modulation of ER stress and apoptosis by endoplasmic reticulum calcium leak via translocon during unfolded protein response: involvement of GRP78. FASEB J 27:1600–1609
Arroba AI, Wallace D, Mackey A, de la Rosa EJ, Cotter TG (2009) IGF-I maintains calpastatin expression and attenuates apoptosis in several models of photoreceptor cell death. Eur J Neurosci 30:975–986
Doonan F, Donovan M, Cotter TG (2003) Caspase-independent photoreceptor apoptosis in mouse models of retinal degeneration. J Neurosci 23:5723–5731
Kaur J, Mencl S, Sahaboglu A, Farinelli P, van Veen T, Zrenner E, Ekström P, Paquet-Durand F et al (2011) Calpain and PARP activation during photoreceptor cell death in P23H and S334ter rhodopsin mutant rats. PLoS One 6:e22181
Paquet-Durand F, Sanges D, McCall J, Silva J, van Veen T, Marigo V, Ekström P (2010) Photoreceptor rescue and toxicity induced by different calpain inhibitors. J Neurochem 115:930–940
Shimazawa M, Suemori S, Inokuchi Y, Matsunaga N, Nakajima Y, Oka T, Yamamoto T, Hara H (2010) A novel calpain inhibitor, ((1S)-1-((((1S)-1-Benzyl-3-cyclopropylamino-2,3-di-oxopropyl)amino)carbonyl)-3-methylbutyl) carbamic acid 5-methoxy-3-oxapentyl ester (SNJ-1945), reduces murine retinal cell death in vitro and in vivo. J Pharmacol Exp Ther 332:380–387
Tzekov R, Stein L, Kaushal S (2011) Protein misfolding and retinal degeneration. Cold Spring Harb Perspect Biol 3:a007492
Sanges D, Marigo V (2006) Cross-talk between two apoptotic pathways activated by endoplasmic reticulum stress: differential contribution of Caspase-12 and AIF. Apoptosis. 11:1629–1641
Petit A, Kawarai T, Paitel E, Sanjo N, Maj M, Scheid M, Chen F, Gu Y et al (2005) Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson disease-related mutations. J Biol Chem 280:34025–34032
Acknowledgments
The authors acknowledge the Cell-lab Facility and CSSI of the University of Modena and Reggio Emilia for the cytofluorimetric analysis and animal husbandry assistance.
Funding
V.M. was supported by the research grant Fondazione Roma (call for proposals 2013 on Retinitis Pigmentosa), European Union (transMed, MSCA-ITN-2017-765441), and Fondazione Telethon (grant numbers GGP11210, GGP14180).
Author information
Authors and Affiliations
Contributions
A.C. and D.S. performed experimental procedures and contributed to the writing of the manuscript. M.M. performed flow cytometry analysis. V. M designed and supervised the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
All procedures on mice were conducted at CSSI (Centro Servizi Stabulario Interdipartimentale), approved by the Ethical Committee of University of Modena and Reggio Emilia and by the Italian Ministero della Salute (346/2015-PR), and were in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Figure S1
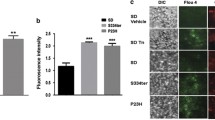
Activation of calpains inRhoP23H/+retinas. (a) Controls for the specificity of the calpain assay. Retina cryosections from PN19 RhoP23H/+ mutant mice were processed for the calpain activity assay either in the absence of t CMAC, t-BOC-Leu-Met substrate (No substrate) or after pretreatment of the crysection with the PD150606 calpain inhibitor (Calpain inh. pretreatment) to block calpain activity. No fluorescent signal could be detected in the control experiments. (b) Retina cryosections from PN15, PN19, PN31 and PN60 wild type (WT) and RhoP23H/+ mutant mice were exposed to a calpain substrate that becomes fluorescent (blue) upon cleavage by calpains. White dots in the outer nuclear layer (onl) containing photoreceptor cells identify cells activating calpains and were detected in mutant retinas but not in WT retinas. The same sections were assayed by TUNEL assay (red) to detect cells undergoing cell death. White dots in the outer nuclear layer (onl) containing photoreceptor cells identify dying cells and were detected in mutant retinas but not in WT retinas. The few PN60 photoreceptor cells undergoing cell death and activating calpains are indicated by arrows. Merged images of calpain activity staining and TUNEL are shown on the right-hand side. Scale bars: 50μm. (PNG 2735 kb)
Figure S2
Validation of the specificity of antibodies used in western blotting experiments. Antibodies used for western blotting to detect activation/phosphorylation of ER-stress sensors P-IRE1, P-PERK and P-eIF2α were validated on protein extracts from the NIH3T3 cell line treated with 2 μg/ml Tunicamycin, for 24 h as in Sanges et al [63]. Antibodies used for western blotting to detect activated/cleaved caspase-7 were validated on protein extracts from NIH3T3 cell line treated with 1 μM Staurosporine for 2 h, as in Petit et al [64]. Antibodies used for western blotting to detect nuclear translocation of NRF2 were validated on protein extracts from the NIH3T3 cell line treated with 5 μg/ml Tunicamycin for 30 min, as in Cullinan et al [37]. (PNG 462 kb)
Figure S4
Cell death analyses on treatedRhoP23H/+retinas. Sections from RhoP23H/+ mutant PN19 retinas either treated with GSK2606414A (PERK inhibitor) or with PD150606 (calpain inhibitor) or Z-VAD-FMK (Caspase inhibitor) or with vehicle (mock) were analyzed by TUNEL assay (red). Nuclei were stained with DAPI (blue) and show all retinal cells. Red dots indicate dying cells. (PNG 470 kb)
Figure S5
Histological analysis of injected eyes. Examples of eyes stained with Hematoxylin and Eosin from not injected and intravitreally injected animals. (PNG 1487 kb)
Rights and permissions
About this article
Cite this article
Comitato, A., Schiroli, D., Montanari, M. et al. Calpain Activation Is the Major Cause of Cell Death in Photoreceptors Expressing a Rhodopsin Misfolding Mutation. Mol Neurobiol 57, 589–599 (2020). https://doi.org/10.1007/s12035-019-01723-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01723-5