Abstract
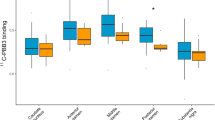
Activated microglia have been reported to play an important role in Parkinson’s disease (PD). A more rapid cognitive decline has been associated with deposits of β-amyloid. In this study, the aim was to evaluate the role of brain β-amyloid and its relationship with activated microglia in PD patients with normal and impaired cognition. We studied 17 PD patients with normal cognition (PDn), 12 PD patients with mild cognitive impairment (PD-MCI), and 12 healthy controls (HCs) with [11C] Pittsburgh compound B (PIB) to assess the impact of β-amyloid deposition in the brain on microglial activation evaluated using the translocator protein 18-kDa (TSPO) radioligand [18F]-FEPPA. [11C] PIB distribution volume ratio was measured in cortical and subcortical regions. [18F]-FEPPA total distribution volume values were compared for each brain region between groups to evaluate the effect of PIB positivity while adjusting for the TSPO rs6971 polymorphism. Factorial analysis of variance revealed a significant main effect of PIB positivity in the frontal lobe (F(1, 34) = 7.1, p = 0.012). Besides the frontal (p = 0.006) and temporal lobe (p = 0.001), the striatum (p = 0.018), the precuneus (p = 0.019), and the dorsolateral prefrontal cortex (p = 0.010) showed significant group × PIB positivity interaction effects. In these regions, PD-MCIs had significantly higher FEPPA VT if PIB-positive. Our results indicate an interaction between amyloid-β deposition and microglial activation in PD. Further investigations are necessary to evaluate if amyloid deposits cause neuroinflammation and further neurodegeneration or if increased microglia activation develops as a protective response.







Similar content being viewed by others
References
Chaudhuri KR, Healy DG, Schapira AHV, National Institute for Clinical Excellence (2006) Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 5:235–245 http://linkinghub.elsevier.com/retrieve/pii/S1474442206703738. Accessed November 4, 2018
Petrou M, Dwamena BA, Foerster BR, MacEachern MP, Bohnen NI, Müller MLTM, Albin RL, Frey KA (2015) Amyloid deposition in Parkinson’s disease and cognitive impairment: a systematic review. Mov Disord 30:928–935
Imamura K, Hishikawa N, Sawada M et al (2003) Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol 106:518–526 http://link.springer.com/10.1007/s00401-003-0766-2. Accessed November 4, 2018
Suridjan I, Pollock BG, Verhoeff NPLG, Voineskos AN, Chow T, Rusjan PM, Lobaugh NJ, Houle S et al (2015) In-vivo imaging of grey and white matter neuroinflammation in Alzheimer’s disease: a positron emission tomography study with a novel radioligand, “18 F”-FEPPA. Mol Psychiatry 20:1579–1587
Edison P, Ahmed I, Fan Z et al (2013) Microglia, amyloid, and glucose metabolism in Parkinson’s disease with and without dementia. Neuropsychopharmacology 38:938–949 http://www.nature.com/articles/npp2012255. Accessed November 4, 2018
Masters CL, Simms G, Weinman NA et al (1985) Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A 82:4245–4249 http://www.ncbi.nlm.nih.gov/pubmed/3159021. Accessed November 4, 2018
Mastaglia FL, Johnsen RD, Byrnes ML et al (2003) Prevalence of amyloid-beta deposition in the cerebral cortex in Parkinson’s disease. Mov Disord 18:81–86 http://doi.wiley.com/10.1002/mds.10295. Accessed November 4, 2018
Petrou M, Bohnen NI, Muller MLTM et al (2012) Abeta-amyloid deposition in patients with Parkinson disease at risk for development of dementia. Neurology 79:1161–1167 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3525303&tool=pmcentrez&rendertype=abstract
Mielke MM, Wiste HJ, Weigand SD, Knopman DS, Lowe VJ, Roberts RO, Geda YE, Swenson-Dravis DM et al (2012) Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology. 79:1570–1577
Edison P, Rowe CC, Rinne JO, Ng S, Ahmed I, Kemppainen N, Villemagne VL, O'Keefe G et al (2008) Amyloid load in Parkinson’s disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatry 79:1331–1338
Gomperts SN, Locascio JJ, Rentz D et al (2013) Amyloid is linked to cognitive decline in patients with Parkinson disease without dementia. Neurology 80:85–91 7p. Accessed at: http://search.ebscohost.com/login.aspx?direct=true&db=ccm&AN=108089057&site=ehost-live
Prokop S, Miller KR, Heppner FL (2013) Microglia actions in Alzheimer’s disease. Acta Neuropathol 126:461–477 http://www.ncbi.nlm.nih.gov/pubmed/24224195. Accessed November 4, 2018
Litvan I, Goldman JG, Tröster AI et al (2012) Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord 27:349–356 http://doi.wiley.com/10.1002/mds.24893. Accessed October 1, 2018
Wilson AA, Garcia A, Parkes J et al (2008) Radiosynthesis and initial evaluation of [18F]-FEPPA for PET imaging of peripheral benzodiazepine receptors. Nucl Med Biol 35:305–314 http://linkinghub.elsevier.com/retrieve/pii/S0969805108000073. Accessed November 4, 2018
Mathis CA, Bacskai BJ, Kajdasz ST et al (2002) A lipophilic thioflavin-T derivative for positron emission tomography (PET) imaging of amyloid in brain. Bioorg Med Chem Lett 12:295–298 http://www.ncbi.nlm.nih.gov/pubmed/11814781. Accessed November 4, 2018
Rusjan PM, Wilson AA, Bloomfield PM, Vitcu I, Meyer JH, Houle S, Mizrahi R (2011) Quantitation of translocator protein binding in human brain with the novel radioligand [18F]-FEPPA and positron emission tomography. J Cereb Blood Flow Metab 31:1807–1816 http://journals.sagepub.com/doi/10.1038/jcbfm.2011.55. Accessed October 1, 2018
Rusjan P, Mamo D, Ginovart N, Hussey D, Vitcu I, Yasuno F, Tetsuya S, Houle S et al (2006) An automated method for the extraction of regional data from PET images. Psychiatry Res - Neuroimaging 147:79–89
Bencherif B, Stumpf MJ, Links JM et al (2004) Application of MRI-based partial-volume correction to the analysis of PET images of mu-opioid receptors using statistical parametric mapping. J Nucl Med 45:402–408 http://www.ncbi.nlm.nih.gov/pubmed/15001679. Accessed November 4, 2018
Lopresti BJ, Klunk WE, Mathis CA et al (2005) Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med 46:1959–1972 http://www.ncbi.nlm.nih.gov/pubmed/16330558. Accessed October 1, 2018
Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, Madison C, Ayakta N, Ghosh PM, la Joie R, Arthur-Bentil SK et al (2015) Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 138:2020–2033
Villemagne VL, Pike KE, Chételat G et al (2011) Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann Neurol 69:181–192 http://doi.wiley.com/10.1002/ana.22248. Accessed October 1, 2018
Lahiri DK, Nurnberger JI (1991) A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res 19:5444 http://www.ncbi.nlm.nih.gov/pubmed/1681511. Accessed November 4, 2018
Mizrahi R, Rusjan PM, Kennedy J, Pollock B, Mulsant B, Suridjan I, de Luca V, Wilson AA et al (2012) Translocator protein (18 kDa) polymorphism (rs6971) explains in-vivo brain binding affinity of the PET radioligand [18F]-FEPPA. J Cereb Blood Flow Metab 32:968–972
Alvarez-Erviti L, Couch Y, Richardson J et al (2011) Alpha-synuclein release by neurons activates the inflammatory response in a microglial cell line. Neurosci Res 69:337–342 http://linkinghub.elsevier.com/retrieve/pii/S0168010211000101. Accessed November 4, 2018
Akhtar RS, Xie SX, Chen YJ, Rick J, Gross RG, Nasrallah IM, van Deerlin VM, Trojanowski JQ et al (2017) Regional brain amyloid-β accumulation associates with domain-specific cognitive performance in Parkinson disease without dementia. PLoS One 12:1–18. https://doi.org/10.1371/journal.pone.0177924
Fan Z, Aman Y, Ahmed I et al (2015) Influence of microglial activation on neuronal function in Alzheimer’s and Parkinson’s disease dementia. Alzheimers Dement 11:608–21.e7 https://linkinghub.elsevier.com/retrieve/pii/S1552526014025011. Accessed November 4, 2018
Gerhard A, Pavese N, Hotton G et al (2006) In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis 21:404–412 http://linkinghub.elsevier.com/retrieve/pii/S0969996105002263. Accessed November 4, 2018
Kobylecki C, Counsell SJ, Cabanel N et al (2013) Diffusion-weighted imaging and its relationship to microglial activation in parkinsonian syndromes. Parkinsonism Relat Disord 19:527–532 https://linkinghub.elsevier.com/retrieve/pii/S1353802013000527. Accessed November 4, 2018
Cai Z, Hussain MD, Yan LJ (2014) Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int J Neurosci 124:307–321
Hamelin L, Lagarde J, Dorothée G, Leroy C, Labit M, Comley RA, de Souza LC, Corne H et al (2016) Early and protective microglial activation in Alzheimer’s disease: a prospective study using18F-DPA-714 PET imaging. Brain. 139:1252–1264
Lavisse S, Guillermier M, Hérard A-S et al (2012) Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. J Neurosci 32:10809–10818 http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.1487-12.2012. Accessed November 4, 2018
Stokholm MG, Iranzo A, Østergaard K et al (2017) Assessment of neuroinflammation in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case-control study. Lancet Neurol 16:789–796 https://linkinghub.elsevier.com/retrieve/pii/S1474442217301734. Accessed November 4, 2018
Stokholm MG, Iranzo A, Østergaard K et al (2018) Extrastriatal monoaminergic dysfunction and enhanced microglial activation in idiopathic rapid eye movement sleep behaviour disorder. Neurobiol Dis 115:9–16 https://linkinghub.elsevier.com/retrieve/pii/S0969996118300494. Accessed November 4, 2018
Jucaite A, Svenningsson P, Rinne JO, Cselényi Z, Varnäs K, Johnström P, Amini N, Kirjavainen A et al (2015) Effect of the myeloperoxidase inhibitor AZD3241 on microglia: a PET study in Parkinson’s disease. Brain 138:2687–2700 https://academic.oup.com/brain/article-lookup/doi/10.1093/brain/awv184. Accessed November 4, 2018
Funding
This study was supported by the National Parkinson Foundation and the Canadian Institutes of Health Research (MOP-136778). Antonio Strafella is supported also by the Canada Research Chair program from the Canadian Institutes of Health Research. Dr. Strafella has received honoraria from GE Healthcare Canada.
Author information
Authors and Affiliations
Contributions
Christine Ghadery: acquisition of data, analysis and interpretation of data, and drafting of the manuscript. Yuko Koshimori: acquisition of data and critical revision of the manuscript for intellectual content. Leigh Christopher: acquisition of data and critical revision of the manuscript for intellectual content. Jinhee Kim: analysis and interpretation of data and critical revision of the manuscript for intellectual content. Pablo Rusjan: methodological PET supervision of data analyses and critical revision of the manuscript for intellectual content. Anthony E. Lang: critical revision of the manuscript for intellectual content. Sylvain Houle: critical revision of the manuscript for intellectual content. Antonio P. Strafella: study concept and design, critical revision of the manuscript for intellectual content, acquisition of funding, and overall study supervision.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declared that they have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghadery, C., Koshimori, Y., Christopher, L. et al. The Interaction Between Neuroinflammation and β-Amyloid in Cognitive Decline in Parkinson’s Disease. Mol Neurobiol 57, 492–501 (2020). https://doi.org/10.1007/s12035-019-01714-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01714-6