Abstract
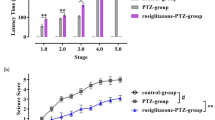
The peroxisome proliferator-activated receptor (PPAR) family, type II nucleus receptors have been successfully tested for their neuroprotective potential in certain central nervous system diseases. The aim of the present study was to determine if modulation by PPAR-γ could attenuate pilocarpine-induced seizures and decrease neuronal excitability. Adult male C57BL/6 mice were divided into two groups: one group received pretreatment with pioglitazone and the other received dimethyl sulfoxide (DMSO) for a period of 2 weeks. Status epilepticus was then induced in both groups by lithium-pilocarpine, after which seizure susceptibility, severity, and mortality were evaluated. Hippocampal histopathology was carried out on all mice at 24 h post-status epilepticus as well as blood–brain barrier (BBB) damage analysis. With the aid of patch clamp technology, the hippocampal neuronal excitability from mice with PPAR-γ 50% expression (PpargC/C) and PPAR-γ 25% expression (PpargC/−), as well as the effect of pioglitazone on the sodium currents in hippocampal neurons, were evaluated. It was found that pioglitazone, a PPAR-γ agonist, could attenuate pilocarpine-induced seizure severity in mice. Pathological examination showed that pioglitazone significantly attenuated pilocarpine-induced status epilepticus-related hippocampal neuronal loss and BBB damage. Further characterization of neuronal excitability revealed higher excitability in the brain slices from mice with PpargC/− expression, compared with the PpargC/C group. It was also found that pioglitazone could decrease sodium currents in hippocampal neurons. In conclusion, PPAR-γ deficiency aggravated neuronal excitability and excitotoxicity. PPAR-γ attenuated pilocarpine-induced seizure severity, neuronal loss, BBB damage, and sodium currents in hippocampal neurons. Modulation of PPAR-γ could be a potential novel treatment for epileptic seizures.





Similar content being viewed by others
References
Sillanpaa M, Schmidt D (2006) Natural history of treated childhood-onset epilepsy: prospective, long-term population-based study. Brain 129:617–624
Kersten S, Desvergne B, Wahli W (2000) Roles of PPARs in health and disease. Nature 405:421–424
Tontonoz P, Spiegelman BM (2008) Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem 77:289–312
Schlachetzki JC, Winkler J (2015) The innate immune system in Parkinson's disease: a novel target promoting endogenous neuroregeneration. Neural Regen Res 10:704–706
Kapadia R, Yi JH, Vemuganti R (2008) Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci 13:813–826
Sundararajan S, Jiang Q, Heneka M, Landreth G (2006) PPARgamma as a therapeutic target in central nervous system diseases. Neurochem Int 49:136–144
Chuang YC, Lin TK, Huang HY, Chang WN, Liou CW, Chen SD, Chang AY, Chan SH (2012) Peroxisome proliferator-activated receptors γ/mitochondrial uncoupling protein 2 signaling protects against seizure-induced neuronal cell death in the hippocampus following experimental status epilepticus. J Neuroinflammation 9:184
Wong SB, Cheng SJ, Hung WC, Lee WT, Min MY (2015) Rosiglitazone suppresses in vitro seizures in hippocampal slice by inhibiting presynaptic glutamate release in a model of temporal lobe epilepsy. PLoS One 10:e0144806
Simeone TA, Matthews SA, Samson KK, Simeone KA (2017) Regulation of brain PPARgamma2 contributes to ketogenic diet anti-seizure efficacy. Exp Neurol 287:54–64
Tsai YS, Kim HJ, Takahashi N, Kim HS, Hagaman JR, Kim JK, Maeda N (2004) Hypertension and abnormal fat distribution but not insulin resistance in mice with P465L PPARgamma. J Clin Invest 114:240–249
Tsai YS, Tsai PJ, Jiang MJ, Chou TY, Pendse A, Kim HS, Maeda N (2009) Decreased PPAR gamma expression compromises perigonadal-specific fat deposition and insulin sensitivity. Mol Endocrinol 23:1787–1798
Chen CY, Chen TM, Shyu AB (1994) Interplay of two functionally and structurally distinct domains of the c-fos AU-rich element specifies its mRNA-destabilizing function. Mol Cell Biol 14:416–426
Tsai YS, Pendse A, Moy SS, Mohri I, Perez A, Crawley JN, Suzuki K, Maeda N (2006) A de novo deafwaddler mutation of Pmca2 arising in ES cells and hitchhiking with a targeted modification of the Pparg gene. Mamm Genome 17:716–722
Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM (1999) PPAR-gamma is required for placental, cardiac, and adipose tissue development. Mol Cell 4:585–595
Huang CW, Cheng JT, Tsai JJ, Wu SN, Huang CC (2009) Diabetic hyperglycemia aggravates seizures and status epilepticus-induced hippocampal damage. Neurotox Res 15:71–81
Huang CW, Wu SN, Cheng JT, Tsai JJ, Huang CC (2010) Diazoxide reduces status epilepticus neuron damage in diabetes. Neurotox Res 17:305–316
Pathak HR, Weissinger F, Terunuma M, Carlson GC, Hsu FC, Moss SJ, Coulter DA (2007) Disrupted dentate granule cell chloride regulation enhances synaptic excitability during development of temporal lobe epilepsy. J Neurosci 27:14012–14022
Ferraro TN, Golden GT, Smith GG, St Jean P, Schork NJ, Mulholland N, Ballas C, Schill J et al (1999) Mapping loci for pentylenetetrazol-induced seizure susceptibility in mice. J Neurosci 19:6733–6739
Chang YC, Huang AM, Kuo YM, Wang ST, Chang YY, Huang CC (2003) Febrile seizures impair memory and cAMP response-element binding protein activation. Ann Neurol 54:706–718
Pitkanen A, Schwartzkroin PA, Moshe SL (2006) Models of seizures and epilepsy. Elsevier Academic Press, Burlington
Huang CW, Lin KM, Hung TY, Chuang YC, Wu SN (2018) Multiple actions of rotenone, an inhibitor of mitochondrial respiratory chain, on ionic currents and miniature end-plate potential in mouse hippocampal (mHippoE-14) neurons. Cell Physiol Biochem 47:330–343
Lin CH, Hsu SP, Cheng TC, Huang CW, Chiang YC, Hsiao IH, Lee MH, Shen ML et al (2017) Effects of anti-epileptic drugs on spreading depolarization-induced epileptiform activity in mouse hippocampal slices. Sci Rep 7:11884
Oby E, Janigro D (2006) The blood–brain barrier and epilepsy. Epilepsia 47:1761–1774
Friedman A, Heinemann U (2012) Role of blood–brain barrier dysfunction in epileptogenesis. In: Noebels JL et al. (eds) Jasper’s basic mechanisms of the epilepsies. National Center for Biotechnology Information (US)
Seiffert E, Dreier JP, Ivens S, Bechmann I, Tomkins O, Heinemann U, Friedman A (2004) Lasting blood–brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci 24:7829–7836
Fabene PF, Navarro Mora G, Martinello M, Rossi B, Merigo F, Ottoboni L, Bach S, Angiari S et al (2008) A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat Med 14:1377–1383
Vezzani A, Granata T (2005) Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia 46:1724–1743
Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, O'Banion K, Klockgether T et al (2005) Acute treatment with the PPARγ agonist pioglitazone and ibuprofen reduces glial inflammation and Aβ1–42 levels in APPV717I transgenic mice. Brain 128:1442–1453
Griggs RB, Donahue RR, Morgenweck J, Grace PM, Sutton A, Watkins LR, Taylor BK (2015) Pioglitazone rapidly reduces neuropathic pain through astrocyte and nongenomic PPARgamma mechanisms. Pain 156:469–482
Hasegawa H, Takano H, Komuro I (2010) Therapeutic implications of PPARgamma in cardiovascular diseases. PPAR Res. https://doi.org/10.1155/2010/876049
Kiss E, Popovic ZV, Bedke J, Adams J, Bonrouhi M, Babelova A, Schmidt C, Edenhofer F et al (2010) Peroxisome proliferator-activated receptor (PPAR)gamma can inhibit chronic renal allograft damage. Am J Pathol 176:2150–2162
Landreth G, Jiang Q, Mandrekar S, Heneka M (2008) PPARgamma agonists as therapeutics for the treatment of Alzheimer’s disease. Neurotherapeutics 5:481–489
Landreth GE, Heneka MT (2001) Anti-inflammatory actions of peroxisome proliferator-activated receptor gamma agonists in Alzheimer’s disease. Neurobiol Aging 22:937–944
Mohazab RA, Javadi-Paydar M, Delfan B, Dehpour AR (2012) Possible involvement of PPAR-gamma receptor and nitric oxide pathway in the anticonvulsant effect of acute pioglitazone on pentylenetetrazole-induced seizures in mice. Epilepsy Res 101:28–35
Okada K, Yamashita U, Tsuji S (2006) Ameliorative effect of pioglitazone on seizure responses in genetically epilepsy-susceptible EL mice. Brain Res 1102:175–178
Vezzani A, French J, Bartfai T, Baram TZ (2011) The role of inflammation in epilepsy. Nat Rev Neurol 7:31–40
Marchi N, Fan Q, Ghosh C, Fazio V, Bertolini F, Betto G, Batra A, Carlton E et al (2009) Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol Dis 33:171–181
Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM (2001) PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med 7:48–52
Park EJ, Park SY, Joe E, Jou I (2003) 15d-PGJ2 and rosiglitazone suppress Janus kinase-STAT inflammatory signaling through induction of suppressor of cytokine signaling 1 (SOCS1) and SOCS3 in glia. J Biol Chem 278:14747–14752
Asano M, Nakajima T, Iwasawa K, Morita T, Nakamura F, Imuta H, Chisaki K, Yamada N et al (1999) Troglitazone and pioglitazone attenuate agonist-dependent Ca2+ mobilization and cell proliferation in vascular smooth muscle cells. Br J Pharmacol 128:673–683
Zhang F, Sowers JR, Ram JL, Standley PR, Peuler JD (1994) Effects of pioglitazone on calcium channels in vascular smooth muscle. Hypertension 24:170–175
Acknowledgments
This work was supported in part by grants from the Taiwan National Science Council (NSC-102-2314-B-006-051-MY3), Ministry of Science and Technology, ROC, (105-2314-B-006-013, 106-2314-B-006-034, 106-2320-B-006-055, 107-2314-B-006-018, 107-2320-B-006-019), National Cheng Kung University Hospital (20180254), and Chi-Mei Medical Center (CMNCKU10303). Part of this abstract was presented at the Taiwan Neurological Society Annual Meeting, 2013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hung, TY., Chu, FL., Wu, D.C. et al. The Protective Role of Peroxisome Proliferator-Activated Receptor-Gamma in Seizure and Neuronal Excitotoxicity. Mol Neurobiol 56, 5497–5506 (2019). https://doi.org/10.1007/s12035-018-1457-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1457-2