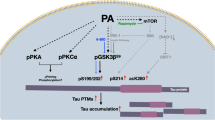
Abstract
Numerous cross-sectional and longitudinal studies have implicated saturated fat-enriched diets in the etio-pathogenesis of Alzheimer’s disease (AD). Emerging evidence shows that saturated fat-enriched diets, such as palmitate-enriched diets, increase amyloid-beta (Aβ) production, the histopathological hallmark of AD. However, the molecular mechanisms that underlie the deleterious effects of palmitate-enriched diets in the augmentation of Aβ genesis are yet to be characterized. Sterol response element binding protein 1 (SREBP1) is a transcription factor that is modulated by saturated fatty acids, such as palmitate, and consequently regulates the expression of genes that code for proteins involved in almost all facets of lipid metabolism. Herein, we determined the role of changes in SREBP1 expression and transcriptional activity in the palmitate-induced effects on Aβ genesis and BACE1 expression, the enzyme that catalyzes the rate-limiting step in Aβ biosynthesis. We demonstrate that palmitate-induced SREBP1 activation directly regulates BACE1 expression at the transcriptional level in the mouse hippocampus and mouse Neuro-2a (N2a) neuroblastoma cells. Chromatin immunoprecipitation (ChIP) studies show that palmitate increases the binding of SREBP1 to the Bace1 promoter region in the mouse hippocampus and mouse N2a neuroblastoma cells. Ectopic expression of the dominant negative SREBP1 mutant and knocking-down SREBP1 expression significantly reduced the palmitate-induced increase in BACE1 expression and subsequent Aβ genesis in mouse N2a neuroblastoma cells. Our study unveils SREBP1 activation as a novel molecular player in the palmitate-induced upregulation of BACE1 expression and subsequent Aβ genesis.





Similar content being viewed by others
Abbreviations
- Aβ:
-
amyloid beta
- AβPP:
-
amyloid beta precursor protein
- AD:
-
Alzheimer’s disease
- BACE1:
-
β-site AβPP cleaving enzyme 1
- CHOP:
-
C/EBP homologous protein
- ChIP:
-
chromatin immunoprecipitation
- ER:
-
endoplasmic reticulum
- LXRα:
-
liver X receptor alpha
- N2a:
-
Neuro-2a mouse neuroblastoma cells
- NCoA:
-
nuclear receptor coactivator
- NFT:
-
neurofibrillary tangles
- PA:
-
palmitic acid
- sFFA:
-
saturated free fatty acids
- SRC:
-
steroid receptor coactivator
- SRE:
-
sterol response element
- SREBP:
-
sterol response element binding protein
- WB:
-
western blotting
References
Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S et al (1999) β-Secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286(5440):735–741. https://doi.org/10.1126/science.286.5440.735
Hardy J, Higgins G (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256(5054):184–185. https://doi.org/10.1126/science.1566067
Fukumoto H, Cheung BS, Hyman BT, Irizarry MC (2002) Β-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol 59(9):1381–1389. https://doi.org/10.1001/archneur.59.9.1381
Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G (2002) Increased expression of the amyloid precursor beta-secretase in Alzheimer’s disease. Ann Neurol 51(6):783–786. https://doi.org/10.1002/ana.10208
Busquets O, Ettcheto M, Pallàs M, Beas-Zarate C, Verdaguer E, Auladell C, Folch J, Camins A (2017) Long-term exposition to a high fat diet favors the appearance of β-amyloid depositions in the brain of C57BL/6J mice. A potential model of sporadic Alzheimer’s disease. Mech Ageing Dev 162:38–45. https://doi.org/10.1016/j.mad.2016.11.002
Thériault P, ElAli A, Rivest S (2016) High fat diet exacerbates Alzheimer’s disease-related pathology in APPswe/PS1 mice. Oncotarget 7(42):67808–67827. https://doi.org/10.18632/oncotarget.12179
Kothari V, Luo Y, Tornabene T, O’Neill AM, Greene MW, Geetha T, Babu JR (2017) High fat diet induces brain insulin resistance and cognitive impairment in mice. Biochim Biophys Acta Mol Basis Dis 1863(2):499–508. https://doi.org/10.1016/j.bbadis.2016.10.006
Janssen CI, Jansen D, Mutsaers MP, Dederen PJ, Geenen B, Mulder MT, Kiliaan AJ (2016) The effect of a high-fat diet on brain plasticity, inflammation and cognition in female ApoE4-knockin and ApoE-knockout mice. PLoS One 11(5):e0155307. https://doi.org/10.1371/journal.pone.0155307
Grant WB (1999) Dietary links to Alzheimer’s disease: 1999 update. J Alzheimers Dis 1(4–5):197–201. https://doi.org/10.3233/JAD-1999-14-501
Solfrizzi V, D’Introno A, Colacicco AM, Capurso C, Del Parigi A, Capurso S, Gadaleta A, Capurso A et al (2005) Dietary fatty acids intake: possible role in cognitive decline and dementia. Exp Gerontol 40(4):257–270. https://doi.org/10.1016/j.exger.2005.01.001
Ulmann L, Mimouni V, Roux S, Porsolt R, Poisson JP (2001) Brain and hippocampus fatty acid composition in phospholipid classes of aged-relative cognitive deficit rats. Prostaglandins Leukot Essent Fat Acids 64(3):189–195. https://doi.org/10.1054/plef.2001.0260
Lovejoy JC, Smith SR, Champagne CM, Most MM, Lefevre M, DeLany JP, Denkins YM, Rood JC et al (2002) Effects of diets enriched in saturated (palmitic), monounsaturated (oleic), or trans (elaidic) fatty acids on insulin sensitivity and substrate oxidation in healthy adults. Diabetes Care 25(8):1283–1288. https://doi.org/10.2337/diacare.25.8.1283
Hamilton JA, Brunaldi K (2007) A model for fatty acid transport into the brain. J Mol Neurosci 33(1):12–17. https://doi.org/10.1007/s12031-007-0050-3
Rapoport SI (2001) In vivo fatty acid incorporation into brain phosholipids in relation to plasma availability, signal transduction and membrane remodeling. J Mol Neurosci 16(2–3):243–261; discussion 279–284. https://doi.org/10.1385/JMN:16:2-3:243
Horton JD, Goldstein JL, Brown MS (2002) SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 109(9):1125–1131. https://doi.org/10.1172/JCI15593
Brown MS, Goldstein JL (1997) The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89(3):331–340. https://doi.org/10.1016/S0092-8674(00)80213-5
Kato T, Shimano H, Yamamoto T, Ishikawa M, Kumadaki S, Matsuzaka T, Nakagawa Y, Yahagi N et al (2008) Palmitate impairs and eicosapentaenoate restores insulin secretion through regulation of SREBP-1c in pancreatic islets. Diabetes 57(9):2382–2392. https://doi.org/10.2337/db06-1806
Natalicchio A, Biondi G, Marrano N, Labarbuta R, Tortosa F, Spagnuolo R, D'Oria R, Carchia E et al (2016) Long-term exposure of pancreatic beta-cells to palmitate results in SREBP-1C-dependent decreases in GLP-1 receptor signaling via CREB and AKT and insulin secretory response. Endocrinology 157(6):2243–2258. https://doi.org/10.1210/en.2015-2003
Vallim T, Salter AM (2010) Regulation of hepatic gene expression by saturated fatty acids. Prostaglandins Leukot Essent Fat Acids 82(4–6):211–218. https://doi.org/10.1016/j.plefa.2010.02.016
Mastrocola R, Guglielmotto M, Medana C, Catalano MG, Cutrupi S, Borghi R, Tamagno E, Boccuzzi G et al (2011) Dysregulation of SREBP2 induces BACE1 expression. Neurobiol Dis 44(1):116–124. https://doi.org/10.1016/j.nbd.2011.06.010
Marwarha G, Schommer J, Lund J, Schommer T, Ghribi O (2018) Palmitate-induced C/EBP homologous protein activation leads to NF-κB-mediated increase in BACE1 activity and amyloid beta genesis. J Neurochem 144(6):761–779. https://doi.org/10.1111/jnc.14292
Marwarha G, Rostad S, Lilek J, Kleinjan M, Schommer J, Ghribi O (2017) Palmitate increases beta-site AbetaPP-cleavage enzyme 1 activity and amyloid-beta genesis by evoking endoplasmic reticulum stress and subsequent C/EBP homologous protein activation. J Alzheimers Dis 57(3):907–925. https://doi.org/10.3233/JAD-161130
Rishi V, Gal J, Krylov D, Fridriksson J, Boysen MS, Mandrup S, Vinson C (2004) SREBP-1 dimerization specificity maps to both the helix-loop-helix and leucine zipper domains: use of a dominant negative. J Biol Chem 279(12):11863–11874. https://doi.org/10.1074/jbc.M308000200
Marwarha G, Claycombe K, Schommer J, Collins D, Ghribi O (2016) Palmitate-induced endoplasmic reticulum stress and subsequent C/EBPalpha homologous protein activation attenuates leptin and insulin-like growth factor 1 expression in the brain. Cell Signal 28(11):1789–1805. https://doi.org/10.1016/j.cellsig.2016.08.012
Marwarha G, Dasari B, Prasanthi JR, Schommer J, Ghribi O (2010) Leptin reduces the accumulation of Abeta and phosphorylated tau induced by 27-hydroxycholesterol in rabbit organotypic slices. J Alzheimers Dis 19(3):1007–1019. https://doi.org/10.3233/JAD-2010-1298
Marwarha G, Dasari B, Prabhakara JP, Schommer J, Ghribi O (2010) beta-Amyloid regulates leptin expression and tau phosphorylation through the mTORC1 signaling pathway. J Neurochem 115(2):373–384. https://doi.org/10.1111/j.1471-4159.2010.06929.x
Marwarha G, Raza S, Prasanthi JR, Ghribi O (2013) Gadd153 and NF-kappaB crosstalk regulates 27-hydroxycholesterol-induced increase in BACE1 and beta-amyloid production in human neuroblastoma SH-SY5Y cells. PLoS One 8(8):e70773. https://doi.org/10.1371/journal.pone.0070773
Marwarha G, Prasanthi JR, Schommer J, Dasari B, Ghribi O (2011) Molecular interplay between leptin, insulin-like growth factor-1, and beta-amyloid in organotypic slices from rabbit hippocampus. Mol Neurodegener 6(1):41. https://doi.org/10.1186/1750-1326-6-41
Marwarha G, Dasari B, Ghribi O (2012) Endoplasmic reticulum stress-induced CHOP activation mediates the down-regulation of leptin in human neuroblastoma SH-SY5Y cells treated with the oxysterol 27-hydroxycholesterol. Cell Signal 24(2):484–492. https://doi.org/10.1016/j.cellsig.2011.09.029
Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS et al (2000) Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev 14(22):2819–2830. https://doi.org/10.1101/gad.844900
Marwarha G, Raza S, Meiers C, Ghribi O (2014) Leptin attenuates BACE1 expression and amyloid-beta genesis via the activation of SIRT1 signaling pathway. Biochim Biophys Acta 1842(9):1587–1595. https://doi.org/10.1016/j.bbadis.2014.05.015
Marwarha G, Claycombe-Larson K, Schommer J, Ghribi O (2017) Maternal low-protein diet decreases brain-derived neurotrophic factor expression in the brains of the neonatal rat offspring. J Nutr Biochem 45:54–66. https://doi.org/10.1016/j.jnutbio.2017.03.005
Barbero-Camps E, Fernandez A, Martinez L, Fernandez-Checa JC, Colell A (2013) APP/PS1 mice overexpressing SREBP-2 exhibit combined Abeta accumulation and tau pathology underlying Alzheimer's disease. Hum Mol Genet 22(17):3460–3476. https://doi.org/10.1093/hmg/ddt201
Marwarha G, Ghribi O (2017) Palmitate-enriched diet-induced ER stress and CHOP activation causes tau hyperphosphorylation in the cultured human neuroblatoma cells and the mouse brain. Alzheimers Dement 13(7):P326. https://doi.org/10.1016/j.jalz.2017.06.036
Liu L, Martin R, Chan C (2013) Palmitate-activated astrocytes via serine palmitoyltransferase increase BACE1 in primary neurons by sphingomyelinases. Neurobiol Aging 34(2):540–550. https://doi.org/10.1016/j.neurobiolaging.2012.05.017
Liu L, Martin R, Kohler G, Chan C (2013) Palmitate induces transcriptional regulation of BACE1 and presenilin by STAT3 in neurons mediated by astrocytes. Exp Neurol 248:482–490. https://doi.org/10.1016/j.expneurol.2013.08.004
Patil S, Melrose J, Chan C (2007) Involvement of astroglial ceramide in palmitic acid-induced Alzheimer-like changes in primary neurons. Eur J Neurosci 26(8):2131–2141. https://doi.org/10.1111/j.1460-9568.2007.05797.x
Patil S, Sheng L, Masserang A, Chan C (2006) Palmitic acid-treated astrocytes induce BACE1 upregulation and accumulation of C-terminal fragment of APP in primary cortical neurons. Neurosci Lett 406(1–2):55–59. https://doi.org/10.1016/j.neulet.2006.07.015
Cao D, Lu H, Lewis TL, Li L (2007) Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J Biol Chem 282(50):36275–36282. https://doi.org/10.1074/jbc.M703561200
Julien C, Tremblay C, Phivilay A, Berthiaume L, Emond V, Julien P, Calon F (2010) High-fat diet aggravates amyloid-beta and tau pathologies in the 3xTg-AD mouse model. Neurobiol Aging 31(9):1516–1531. https://doi.org/10.1016/j.neurobiolaging.2008.08.022
Maesako M, Uemura K, Kubota M, Kuzuya A, Sasaki K, Asada M, Watanabe K, Hayashida N et al (2012) Environmental enrichment ameliorated high-fat diet-induced Abeta deposition and memory deficit in APP transgenic mice. Neurobiol Aging 33(5):1011 e1011–1011 e1023. https://doi.org/10.1016/j.neurobiolaging.2011.10.028
Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K et al (2000) Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis 7(4):321–331. https://doi.org/10.1006/nbdi.2000.0304
Thirumangalakudi L, Prakasam A, Zhang R, Bimonte-Nelson H, Sambamurti K, Kindy MS, Bhat NR (2008) High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J Neurochem 106(1):475–485. https://doi.org/10.1111/j.1471-4159.2008.05415.x
Vandal M, White PJ, Tremblay C, St-Amour I, Chevrier G, Emond V, Lefrancois D, Virgili J et al (2014) Insulin reverses the high-fat diet-induced increase in brain Abeta and improves memory in an animal model of Alzheimer disease. Diabetes 63(12):4291–4301. https://doi.org/10.2337/db14-0375
Ghribi O (2008) Potential mechanisms linking cholesterol to Alzheimer’s disease-like pathology in rabbit brain, hippocampal organotypic slices, and skeletal muscle. J Alzheimers Dis 15(4):673–684. https://doi.org/10.3233/JAD-2008-15412
Marwarha G, Ghribi O (2015) Does the oxysterol 27-hydroxycholesterol underlie Alzheimer’s disease-Parkinson’s disease overlap? Exp Gerontol 68:13–18. https://doi.org/10.1016/j.exger.2014.09.013
Selvi Y, Gergerlioglu HS, Akbaba N, Oz M, Kandeger A, Demir EA, Yerlikaya FH, Nurullahoglu-Atalik KE (2016) Impact of enriched environment on production of tau, amyloid precursor protein and, amyloid-beta peptide in high-fat and high-sucrose-fed rats. Acta Neuropsychiatr 1–8. doi:https://doi.org/10.1017/neu.2016.63
Ghribi O, Marwarha G (2010) Cholesterol causes Alzheimer pathology through Akt/mTOR inhibition. Alzheimers Dement 6(4):S402–S403. https://doi.org/10.1016/j.jalz.2010.05.1355
Marwarha G, Raza S, Hammer K, Ghribi O (2017) 27-hydroxycholesterol: a novel player in molecular carcinogenesis of breast and prostate cancer. Chem Phys Lipids. https://doi.org/10.1016/j.chemphyslip.2017.05.012
Hannah VC, Ou J, Luong A, Goldstein JL, Brown MS (2001) Unsaturated fatty acids down-regulate srebp isoforms 1a and 1c by two mechanisms in HEK-293 cells. J Biol Chem 276(6):4365–4372
Russo GL (2009) Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem Pharmacol 77(6):937–946
Barnard ND, Bunner AE, Agarwal U (2014) Saturated and trans fats and dementia: a systematic review. Neurobiol Aging 35(Suppl 2):S65–S73. https://doi.org/10.1016/j.neurobiolaging.2014.02.030
Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM (1997) Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol 42(5):776–782. https://doi.org/10.1002/ana.410420514
Laitinen MH, Ngandu T, Rovio S, Helkala EL, Uusitalo U, Viitanen M, Nissinen A, Tuomilehto J et al (2006) Fat intake at midlife and risk of dementia and Alzheimer’s disease: a population-based study. Dement Geriatr Cogn Disord 22(1):99–107. https://doi.org/10.1159/000093478
Luchsinger JA, Tang MX, Shea S, Mayeux R (2002) Caloric intake and the risk of Alzheimer disease. Arch Neurol 59(8):1258–1263. https://doi.org/10.1001/archneur.59.8.1258
Parrott MD, Greenwood CE (2007) Dietary influences on cognitive function with aging: from high-fat diets to healthful eating. Ann N Y Acad Sci 1114:389–397. https://doi.org/10.1196/annals.1396.028
Winocur G, Greenwood CE (1999) The effects of high fat diets and environmental influences on cognitive performance in rats. Behav Brain Res 101(2):153–161. https://doi.org/10.1016/S0166-4328(98)00147-8
Winocur G, Greenwood CE (2005) Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol Aging 26(Suppl 1):46–49. https://doi.org/10.1016/j.neurobiolaging.2005.09.003
Wang H, Kouri G, Wollheim CB (2005) ER stress and SREBP-1 activation are implicated in beta-cell glucolipotoxicity. J Cell Sci 118(Pt 17):3905–3915. https://doi.org/10.1242/jcs.02513
Basseri S, Austin RC (2012) Endoplasmic reticulum stress and lipid metabolism: mechanisms and therapeutic potential. Biochem Res Int 2012:841362. https://doi.org/10.1155/2012/841362
Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferre P, Foufelle F (2009) GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest 119(5):1201–1215. https://doi.org/10.1172/JCI37007
Marwarha G, Ghribi O (2018) Saturated fat-enriched diet decreases SIRT1 expression in the mouse hippocampus - the SIRTain effects of saturated fat in the brain. FASEB J 32(1_supplement):lb7. https://doi.org/10.1096/fasebj.2018.32.1_supplement.lb7
Ponugoti B, Kim D-H, Xiao Z, Smith Z, Miao J, Zang M, Wu S-Y, Chiang C-M et al (2010) SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem 285(44):33959–33970. https://doi.org/10.1074/jbc.M110.122978
Marwarha G, Ghribi O (2018) Leptin alleviates the saturated fatty acid-induced increase in BACE1 expression and amyloid-β production - relevance to Alzheimer’s disease pathogenesis. FASEB J 32(1_supplement):659.652. https://doi.org/10.1096/fasebj.2018.32.1_supplement.659.2
Marwarha G, Ghribi O (2012) Leptin signaling and Alzheimer’s disease. Am J Neurodegener Dis 1(3):245–265
Marwarha G, Ghribi O (2012) Cellular model of Alzheimer’s disease--relevance to therapeutic testing. Exp Neurol 233(2):733–739. https://doi.org/10.1016/j.expneurol.2011.11.011
Marwarha GSA (2011) Leptin expression and signaling at the confluence of neurodegenerative mechanisms in Alzheimer disease. The University of North Dakota ProQuest Dissertations Publishing 3515504
Marwarha G (2011) Mutual upregulation of IGF-1 and leptin expression prevents their β-amyloid-induced down regulation. Alzheimers Dement 7(4):S586. https://doi.org/10.1016/j.jalz.2011.05.1659
Nogalska A, Sucajtys-Szulc E, Swierczynski J (2005) Leptin decreases lipogenic enzyme gene expression through modification of SREBP-1c gene expression in white adipose tissue of aging rats. Metabolism 54(8):1041–1047. https://doi.org/10.1016/j.metabol.2005.03.007
Bedi S, Hines GV, Lozada-Fernandez VV, de Jesus Piva C, Kaliappan A, Rider SD Jr, Hostetler HA (2017) Fatty acid binding profile of the liver X receptor alpha. J Lipid Res 58(2):393–402. https://doi.org/10.1194/jlr.M072447
Tobin KA, Steineger HH, Alberti S, Spydevold O, Auwerx J, Gustafsson JA, Nebb HI (2000) Cross-talk between fatty acid and cholesterol metabolism mediated by liver X receptor-alpha. Mol Endocrinol 14(5):741–752. https://doi.org/10.1210/mend.14.5.0459
Hong C, Tontonoz P (2014) Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat Rev Drug Discov 13(6):433–444. https://doi.org/10.1038/nrd4280
Calkin AC, Tontonoz P (2012) Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol 13(4):213–224. https://doi.org/10.1038/nrm3312
Marwarha G, Ghribi O (2017) Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) – a friend, a foe, or a bystander - in the neurodegenerative cascade and pathogenesis of Alzheimer’s disease. CNS Neurol Disord Drug Targets 16(10):1050–1065. https://doi.org/10.2174/1871527316666170725114652
Funding
This work was supported by a Grant from the National Institute of Health (R01AG045264) to Dr. Othman Ghribi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval of Animal Studies
All animal procedures and studies carried out were approved by the Institutional Animal Care and Use Committee at the University of North Dakota. All animal procedures and studies were carried out in accordance with the U.S Public Health Service’s “Policy on Humane Care and Use of Laboratory Animals” and “Guide for the Care and Use of Laboratory Animals”. All animal procedures and studies carried out are in compliance with the U.S National Research Council’s “Guide for the Care and Use of Laboratory Animals.”
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marwarha, G., Claycombe-Larson, K., Lund, J. et al. Palmitate-Induced SREBP1 Expression and Activation Underlies the Increased BACE 1 Activity and Amyloid Beta Genesis. Mol Neurobiol 56, 5256–5269 (2019). https://doi.org/10.1007/s12035-018-1451-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1451-8