Abstract
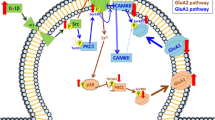
Chronic hyperammonemia impairs spatial memory by altering membrane expression of GluA1 and GluA2 subunits of AMPA receptors in hippocampus. Intracerebral administration of extracellular cGMP to hyperammonemic rats restores spatial memory and membrane expression of AMPA receptors. The underlying molecular mechanisms remain unknown and cannot be analyzed in vivo. The aims of the present work were to (1) assess whether extracellular cGMP reverses the alterations in membrane expression of GluA1 and GluA2 in hippocampus of hyperammonemic rats ex vivo and (2) identify the underlying mechanisms. To reach these aims, we used freshly isolated hippocampal slices from control and hyperammonemic rats and treated them ex vivo with extracellular cGMP. Extracellular cGMP normalizes membrane expression of GluA2 restoring its phosphorylation in Ser880 because it restores PKCζ activation by Thr560 auto-phosphorylation, which is a consequence of normalization by extracellular cGMP of phosphorylation and activity of p38 which was increased in hyperammonemic rats. Normalization of p38 is a consequence of normalization of membrane expression of the GluN2B subunit of NMDA receptor, mediated by a reduction in its phosphorylation in Tyr1472 due to reduction of Src activation, which was over-activated in hyperammonemic rats. Extracellular cGMP also restores membrane expression of GluA1 increasing its phosphorylation at Ser831 because it restores CaMKII membrane association and phosphorylation in Thr286. All these effects of extracellular cGMP are due to a reduction of hippocampal IL-1β levels in hyperammonemic rats, which reduces IL-1 receptor-mediated Src over-activation. Reduction in IL-1β levels is due to the reduction of microglia activation in hippocampus of hyperammonemic rats.






Similar content being viewed by others
References
Felipo V (2013) Hepatic encephalopathy: effects of liver failure on brain function. Nat Rev Neurosci 14(12):851–858. https://doi.org/10.1038/nrn3587
Felipo V, Ordoño JF, Urios A, El Mlili N, Giménez-Garzó C, Aguado C et al (2012) Patients with minimal hepatic encephalopathy show impaired mismatch negativity correlating with reduced performance in attention tests. Hepatology 55(2):530–539. https://doi.org/10.1002/hep.24704
Liao L-M, Zhou L-X, Le H-B, Yin J-J, Ma S-H (2012) Spatial working memory dysfunction in minimal hepatic encephalopathy: an ethology and BOLD-fMRI study. Brain Res 22(1445):62–72. https://doi.org/10.1016/j.brainres.2012.01.036
Schiff S, D’Avanzo C, Cona G, Goljahani A, Montagnese S, Volpato C et al (2014) Insight into the relationship between brain/behavioral speed and variability in patients with minimal hepatic encephalopathy. Clin Neurophysiol 1 125(2):287–297. https://doi.org/10.1016/j.clinph.2013.08.004
Leevy CB, Phillips JA (2007) Hospitalizations during the use of rifaximin versus lactulose for the treatment of hepatic encephalopathy. Dig Dis Sci 1 52(3):737–741. https://doi.org/10.1007/s10620-006-9442-4
Felipo V, Butterworth RF (2002) Neurobiology of ammonia. Prog Neurobiol 67(4):259–279. https://doi.org/10.1016/S0301-0082(02)00019-9
Felipo V, Urios A, Montesinos E, Molina I, Garcia-Torres ML, Civera M et al (2012) Contribution of hyperammonemia and inflammatory factors to cognitive impairment in minimal hepatic encephalopathy. Metab Brain Dis 1 27(1):51–58. https://doi.org/10.1007/s11011-011-9269-3
Iwasa M, Takei Y (2015) Pathophysiology and management of hepatic encephalopathy 2014 update: ammonia toxicity and hyponatremia. Hepatol Res 45(12):1155–1162. https://doi.org/10.1111/hepr.12495
Rahimi RS, Rockey DC (2015) Novel ammonia-lowering agents for hepatic encephalopathy. Clin Liver Dis 1 19(3):539–549. https://doi.org/10.1016/j.cld.2015.04.008
Hernández-Rabaza V, Cabrera-Pastor A, Taoro-González L, Malaguarnera M, Agustí A, Llansola M et al (2016) Hyperammonemia induces glial activation, neuroinflammation and alters neurotransmitter receptors in hippocampus, impairing spatial learning: reversal by sulforaphane. J Neuroinf 16(13):41. https://doi.org/10.1186/s12974-016-0505-y
Cabrera-Pastor A, Hernandez-Rabaza V, Taoro-Gonzalez L, Balzano T, Llansola M, Felipo V (2016) In vivo administration of extracellular cGMP normalizes TNF-α and membrane expression of AMPA receptors in hippocampus and spatial reference memory but not IL-1β, NMDA receptors in membrane and working memory in hyperammonemic rats. Brain Behav Immun 1(57):360–370. https://doi.org/10.1016/j.bbi.2016.05.011
Johansson M, Agusti A, Llansola M, Montoliu C, Strömberg J, Malinina E et al (2015) GR3027 antagonizes GABAA receptor-potentiating neurosteroids and restores spatial learning and motor coordination in rats with chronic hyperammonemia and hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol 2 309(5):G400–G409. https://doi.org/10.1152/ajpgi.00073.2015
Llansola M, Montoliu C, Cauli O, Hernández-Rabaza V, Agustí A, Cabrera-Pastor A et al (2013) Chronic hyperammonemia, glutamatergic neurotransmission and neurological alterations. Metab Brain Dis 1 28(2):151–154. https://doi.org/10.1007/s11011-012-9337-3
Monfort P, Muñoz M-D, ElAyadi A, Kosenko E, Felipo V (2002) Effects of hyperammonemia and liver failure on glutamatergic neurotransmission. Metab Brain Dis 1 17(4):237–250. https://doi.org/10.1523/JNEUROSCI.22-23-10116.2002
Cauli O, Rodrigo R, Llansola M, Montoliu C, Monfort P, Piedrafita B et al (2009) Glutamatergic and gabaergic neurotransmission and neuronal circuits in hepatic encephalopathy. Metab Brain Dis 1 24(1):69–80. https://doi.org/10.1007/s11011-008-9115-4
Llansola M, Montoliu C, Agusti A, Hernandez-Rabaza V, Cabrera-Pastor A, Gomez-Gimenez B et al (2015) Interplay between glutamatergic and GABAergic neurotransmission alterations in cognitive and motor impairment in minimal hepatic encephalopathy. Neurochem Int 1 88:15–19. https://doi.org/10.1016/j.neuint.2014.10.011
Erceg S, Monfort P, Hernandez-Viadel M, Llansola M, Montoliu C, Felipo V (2005) Restoration of learning ability in hyperammonemic rats by increasing extracellular cGMP in brain. Brain Res 2 1036(1):115–121. https://doi.org/10.1016/j.brainres.2004.12.045
Cabrera-Pastor A, Balzano T, Hernández-Rabaza V, Malaguarnera M, Llansola M, Felipo V (2018) Increasing extracellular cGMP in cerebellum in vivo reduces neuroinflammation, GABAergic tone and motor in-coordination in hyperammonemic rats. Brain Behav Immun 1 69:386–398. https://doi.org/10.1016/j.bbi.2017.12.013
Taoro-Gonzalez L, Arenas YM, Cabrera-Pastor A, Felipo V (2018) Hyperammonemia alters membrane expression of GluA1 and GluA2 subunits of AMPA receptors in hippocampus by enhancing activation of the IL-1 receptor: underlying mechanisms. J Neuroinf 8 15:36. https://doi.org/10.1186/s12974-018-1082-z
Esteban JA, Shi S-H, Wilson C, Nuriya M, Huganir RL, Malinow R (2003) PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci 6(2):136–143. https://doi.org/10.1038/nn997
Man H-Y, Sekine-Aizawa Y, Huganir RL (2007) Regulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. PNAS 27 104(9):3579–3584. https://doi.org/10.1073/pnas.0611698104
Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL (1996) Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 1 16(6):1179–1188. https://doi.org/10.1016/S0896-6273(00)80144-0
Mammen AL, Kameyama K, Roche KW, Huganir RL (1997) Phosphorylation of the α-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem 19 272(51):32528–32533. https://doi.org/10.1074/jbc.272.51.32528
Lee H-K, Kameyama K, Huganir RL, Bear MF (1998) NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron 1;21(5):1151–1162. https://doi.org/https://doi.org/10.1016/S0896-6273(00)80632-7
Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL (2000) Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci 1 20(19):7258–7267. https://doi.org/10.1523/JNEUROSCI.20-19-07258.2000
Perez JL, Khatri L, Chang C, Srivastava S, Osten P, Ziff EB (2001) PICK1 targets activated protein kinase C alpha to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. J Neurosci 1 21(15):5417–5428. https://doi.org/10.1523/JNEUROSCI.21-15-05417.2001
Qin Y, Zhu Y, Baumgart JP, Stornetta RL, Seidenman K, Mack V, van Aelst L, Zhu JJ (2005) State-dependent Ras signaling and AMPA receptor trafficking. Genes Dev 19(17):2000–2015. https://doi.org/10.1101/gad.342205
Matsuda S, Mikawa S, Hirai H (1999) Phosphorylation of Serine-880 in GluR2 by protein kinase C prevents its C terminus from binding with glutamate receptor-interacting protein. J Neurochem 73(4):1765–1768. https://doi.org/10.1046/j.1471-4159.1999.731765.x
Kim JS, Park ZY, Yoo YJ, Yu SS, Chun JS (2005) p38 kinase mediates nitric oxide-induced apoptosis of chondrocytes through the inhibition of protein kinase C ζ by blocking autophosphorylation. Cell Death Differ 12:201–212. https://doi.org/10.1038/sj.cdd.4401511
Li S, Tian X, Hartley DM, Feig LA (2006) Distinct roles for Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) and Ras-GRF2 in the induction of long-term potentiation and long-term depression. J Neurosci 1 26(6):1721–1729. https://doi.org/10.1523/JNEUROSCI.3990-05.2006
Viviani B, Gardoni F, Bartesaghi S, Corsini E, Facchi A, Galli CL et al (2006) Interleukin-1β released by gp120 drives neural death through tyrosine phosphorylation and trafficking of NMDA receptors. J Biol Chem 10 281(40):30212–30222. https://doi.org/10.1074/jbc.M602156200
Shaftel SS, Griffin WST, O’Banion MK (2008) The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinf 26 5:7. https://doi.org/10.1186/1742-2094-5-7
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19(8):312–318. https://doi.org/10.1016/0166-2236(96)10049-7
Kettenmann H, Hanisch UK, Noda M & Verkhratsky A (2011). Physiology of microglia. Physiol Rev, 91(2), 461–553. https://doi.org/https://doi.org/10.1152/physrev.00011.2010
Hovens IB, Nyakas C, Schoemaker RG (2014) A novel method for evaluating microglial activation using ionized calcium-binding adaptor protein-1 staining: cell body to cell size ratio. Neurol Neuroimmunol Neuroinflamm 1:82. https://doi.org/10.4103/2347-8659.139719
Rodrigo R, Cauli O, Gomez-Pinedo U, Agusti A, Hernandez-Rabaza V, Garcia-Verdugo J-M et al (2010) Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology 1 139(2):675–684. https://doi.org/10.1053/j.gastro.2010.03.040
Ugarte A, Gil-Bea F, García-Barroso C, Cedazo-Minguez Á, Ramírez MJ, Franco R, García-Osta A, Oyarzabal J et al (2015) Decreased levels of guanosine 3′, 5′-monophosphate (cGMP) in cerebrospinal fluid (CSF) are associated with cognitive decline and amyloid pathology in Alzheimer’s disease. Neuropathol Appl Neurobiol 41(4):471–482. https://doi.org/10.1111/nan.12203
Oeckl P, Steinacker P, Lehnert S, Jesse S, Kretzschmar HA, Ludolph AC et al (2012) CSF concentrations of cAMP and cGMP are lower in patients with Creutzfeldt-Jakob disease but not Parkinson’s disease and amyotrophic lateral sclerosis. Plos One 2 7(3):e32664. https://doi.org/10.1371/journal.pone.0032664
Cuadrado-Tejedor M, Hervias I, Ricobaraza A, Puerta E, Pérez-Roldán JM, García-Barroso C, Franco R, Aguirre N et al (2011) Sildenafil restores cognitive function without affecting β-amyloid burden in a mouse model of Alzheimer’s disease. Brit J Pharmacol 164(8):2029–2041. https://doi.org/10.1111/j.1476-5381.2011.01517.x
García-Barroso C, Ricobaraza A, Pascual-Lucas M, Unceta N, Rico AJ, Goicolea MA et al (2013) Tadalafil crosses the blood–brain barrier and reverses cognitive dysfunction in a mouse model of AD. Neuropharmacology 1 64:114–123. https://doi.org/10.1016/j.neuropharm.2012.06.052
Hernandez-Rabaza V, Agusti A, Cabrera-Pastor A, Fustero S, Delgado O, Taoro-Gonzalez L et al (2015) Sildenafil reduces neuroinflammation and restores spatial learning in rats with hepatic encephalopathy: underlying mechanisms. J Neuroinflamm 29(12):195. https://doi.org/10.1186/s12974-015-0420-7
Saavedra A, Giralt A, Arumí H, Alberch J, Pérez-Navarro E (2013) Regulation of hippocampal cGMP levels as a candidate to treat cognitive deficits in Huntington’s disease. Plos One 5 8(9):e73664. https://doi.org/10.1371/journal.pone.0073664
Paris D, Town T, Parker T, Humphrey J, Mullan M (2000) Beta-Amyloid vasoactivity and proinflammation in microglia can be blocked by cGMP-elevating agents. Ann N Y Acad Sci 903:446–450. https://doi.org/10.1111/j.1749-6632.2000.tb06397.x
Yoshioka Y, Takeda N, Yamamuro A, Kasai A, Maeda S (2010) Nitric oxide inhibits lipopolysaccharide-induced inducible nitric oxide synthase expression and its own production through the cGMP signalling pathway in murine microglia BV-2 cells. J Pharmacol Sci 113(2):153–160. https://doi.org/10.1254/jphs.10060FP
Moretti R, Leger PL, Besson VC, Csaba Z, Pansiot J, Di Criscio L, Gentili A, Titomanlio L et al (2016) Sildenafil, a cyclic GMP phosphodiesterase inhibitor, induces microglial modulation after focal ischemia in the neonatal mouse brain. J Neuroinflamm 28 13(1):95. https://doi.org/10.1186/s12974-016-0560-4
Agusti A, Hernández-Rabaza V, Balzano T, Taoro-Gonzalez L, Ibañez-Grau A, Cabrera-Pastor A, Fustero S, Llansola M et al (2017) Sildenafil reduces neuroinflammation in cerebellum, restores GABAergic tone and improves motor in-coordination in rats with hepatic encephalopathy. CNS Neurosci Ther 23(5):386–394. https://doi.org/10.1111/cns.12688
Choi SH, Choi DH, Song KS, Shin KH, Chun BG (2002) Zaprinast, an inhibitor of cGMP-selective phosphodiesterases, enhances the secretion of TNF-alpha and IL-1beta and the expression of iNOS and MHC class II molecules in rat microglial cells. J Neurosci Res 1 67(3):411–421. https://doi.org/10.1002/jnr.10102
Cabrera-Pastor A, Balzano T, Hernández-Rabaza V, Malaguarnera M, Llansola M, Felipo V (2018) Increasing extracellular cGMP in cerebellum in vivo reduces neuroinflammation, GABAergic tone and motor in-coordination in hyperammonemic rats. Brain Behav Immun 69:386–398. https://doi.org/10.1016/j.bbi.2017.12.013
Felipo V, Miñana M-D, Grisolía S (1988) Long-term ingestion of ammonium increases acetylglutamate and urea levels without affecting the amount of carbamoyl-phosphate synthase. Eur J Biochem 176(3):567–571. https://doi.org/10.1111/j.1432-1033.1988.tb14315.x
Felipo V, Grau E, Miñana M-D, Grisolía S (1993) Ammonium injection induces an N-methyl-d-aspartate receptor-mediated proteolysis of the microtubule-associated protein MAP-2. J Neurochem 60(5):1626–1630. https://doi.org/10.1111/j.1471-4159.1993.tb13384.x
Cabrera-Pastor A, Taoro L, Llansola M, Felipo V (2015) Roles of the NMDA receptor and EAAC1 transporter in the modulation of extracellular glutamate by low and high affinity AMPA receptors in the cerebellum in vivo: differential alteration in chronic hyperammonemia. ACS Chem Neurosci 16 6(12):1913–1921. https://doi.org/10.1021/acschemneuro.5b00212
Funding
Ministerio de Ciencia e Innovación Spain (SAF2014-51851-R and SAF2017-82917-R), Consellería Educación Generalitat Valenciana (PROMETEOII/2014/033), co-funded with European Regional Development Funds (ERDF); Ministerio de Educación, Cultura y Deporte (FPU13/02492).
Author information
Authors and Affiliations
Contributions
LTG: most ex vivo experiments with fresh slices, membrane expression experiments and western blot analysis; LTG and ACP: analysis and interpretation of data, drafting of the manuscript; YMA: immunohistochemical studies and analysis; ACP and VF: study concept, design and supervision, analysis and interpretation of data; VF: obtained funding, writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Taoro-Gonzalez, L., Arenas, Y.M., Cabrera-Pastor, A. et al. Extracellular cGMP Reverses Altered Membrane Expression of AMPA Receptors in Hippocampus of Hyperammonemic Rats: Underlying Mechanisms. Mol Neurobiol 56, 4428–4439 (2019). https://doi.org/10.1007/s12035-018-1387-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1387-z