Abstract
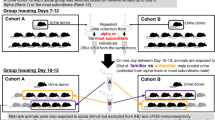
The basolateral amygdala (BLA) is a critical nucleus mediating behavioral responses after exposure to acute social conflict. Male and female Syrian hamsters both readily establish a stable dominant-subordinate relationship among same-sex conspecifics, and the goal of the current study was to determine potential underlying genetic mechanisms in the BLA facilitating the establishment of social hierarchy. We sequenced the BLA transcriptomes of dominant, subordinate, and socially neutral males and females, and using de novo assembly techniques and gene network analyses, we compared these transcriptomes across social status within each sex. Our results revealed 499 transcripts that were differentially expressed in the BLA across both males and females and 138 distinct gene networks. Surprisingly, we found that there was virtually no overlap in the transcript changes or in gene network patterns in males and females of the same social status. These results suggest that, although males and females reliably engage in similar social behaviors to establish social dominance, the molecular mechanisms in the BLA by which these statuses are obtained and maintained are distinct.






Similar content being viewed by others
References
Bjorkqvist K (2001) Social defeat as a stressor in humans. Physiol Behav 73(3):435–442
Agid O, Kohn Y, Lerer B (2000) Environmental stress and psychiatric illness. Biomed Pharmacother 54(3):135–141. https://doi.org/10.1016/s0753-3322(00)89046-0
Ehlers A, Maercker A, Boos A (2000) Posttraumatic stress disorder following political imprisonment: The role of mental defeat, alienation, and perceived permanent change. J Abnorm Psychol 109(1):45–55
Kelleher I, Harley M, Lynch F, Arseneault L, Fitzpatrick C, Cannon M (2008) Associations between childhood trauma, bullying and psychotic symptoms among a school-based adolescent sample. Br J Psychiatry J Ment Sci 193(5):378–382. https://doi.org/10.1192/bjp.bp.108.049536
Borghans B, Homberg JR (2015) Animal models for posttraumatic stress disorder: an overview of what is used in research. World J Psychiatry 5(4):387–396. https://doi.org/10.5498/wjp.v5.i4.387
Tamashiro KL, Nguyen MM, Sakai RR (2005) Social stress: from rodents to primates. Front Neuroendocrinol 26(1):27–40. https://doi.org/10.1016/j.yfrne.2005.03.001
Huhman KL (2006) Social conflict models: can they inform us about human psychopathology? Horm Behav 50(4):640–646. https://doi.org/10.1016/j.yhbeh.2006.06.022
Krishnan V (2014) Defeating the fear: new insights into the neurobiology of stress susceptibility. Exp Neurol 261:412–416. https://doi.org/10.1016/j.expneurol.2014.05.012
Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA (1991) Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav 38(2):315–320
Toth I, Neumann ID (2013) Animal models of social avoidance and social fear. Cell Tissue Res 354(1):107–118. https://doi.org/10.1007/s00441-013-1636-4
Chaouloff F (2013) Social stress models in depression research: what do they tell us? Cell Tissue Res 354(1):179–190. https://doi.org/10.1007/s00441-013-1606-x
Hollis F, Kabbaj M (2014) Social defeat as an animal model for depression. ILAR J 55(2):221–232. https://doi.org/10.1093/ilar/ilu002
Altemus M (2006) Sex differences in depression and anxiety disorders: potential biological determinants. Horm Behav 50(4):534–538
Breslau N, Davis GC, Andreski P, Peterson EL, Schultz LR (1997) Sex differences in posttraumatic stress disorder. Arch Gen Psychiatry 54(11):1044–1048
Nolen-Hoeksema S (1987) Sex differences in unipolar depression: evidence and theory. Psychol Bull 101(2):259–282
Weissman MM, Klerman GL (1977) Sex differences and the epidemiology of depression. Arch Gen Psychiatry 34(1):98–111
St John RD, Corning PA (1973) Maternal aggression in mice. Behav Biol 9(5):635–639
Hennessey AC, Huhman KL, Albers HE (1994) Vasopressin and sex differences in hamster flank marking. Physiol Behav 55(5):905–911
Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM (2003) Conditioned defeat in male and female Syrian hamsters. Horm Behav 44(3):293–299
Taravosh-Lahn K, Delville Y (2004) Aggressive behavior in female golden hamsters: development and the effect of repeated social stress. Horm Behav 46(4):428–435. https://doi.org/10.1016/j.yhbeh.2004.03.007
Rosenhauer AM, McCann KE, Norvelle A, Huhman KL (2017) An acute social defeat stressor in early puberty increases susceptibility to social defeat in adulthood. Horm Behav 93:31–38. https://doi.org/10.1016/j.yhbeh.2017.04.002
Faruzzi AN, Solomon MB, Demas GE, Huhman KL (2005) Gonadal hormones modulate the display of submissive behavior in socially defeated female Syrian hamsters. Horm Behav 47(5):569–575. https://doi.org/10.1016/j.yhbeh.2004.11.023
Solomon MB, Karom MC, Huhman KL (2007) Sex and estrous cycle differences in the display of conditioned defeat in Syrian hamsters. Horm Behav 52(2):211–219. https://doi.org/10.1016/j.yhbeh.2007.04.007
Solomon MB, Karom MC, Norvelle A, Markham CA, Erwin WD, Huhman KL (2009) Gonadal hormones modulate the display of conditioned defeat in male Syrian hamsters. Horm Behav 56(4):423–428. https://doi.org/10.1016/j.yhbeh.2009.07.011
McCann KE, Rosenhauer AM, Jones GMF, Norvelle A, Choi DC, Huhman KL (2017) Histone deacetylase and acetyltransferase inhibitors modulate behavioral responses to social stress. Psychoneuroendocrinology 75:100–109. https://doi.org/10.1016/j.psyneuen.2016.10.022
Jasnow AM, Huhman KL (2001) Activation of GABA(A) receptors in the amygdala blocks the acquisition and expression of conditioned defeat in Syrian hamsters. Brain Res 920(1–2):142–150
Markham CM, Taylor SL, Huhman KL (2010) Role of amygdala and hippocampus in the neural circuit subserving conditioned defeat in Syrian hamsters. Learn Mem 17(2):109–116. https://doi.org/10.1101/lm.1633710
Markham CM, Huhman KL (2008) Is the medial amygdala part of the neural circuit modulating conditioned defeat in Syrian hamsters? Learn Mem 15(1):6–12. https://doi.org/10.1101/lm.768208
Jasnow AM, Shi C, Israel JE, Davis M, Huhman KL (2005) Memory of social defeat is facilitated by cAMP response element-binding protein overexpression in the amygdala. Behav Neurosci 119(4):1125–1130. https://doi.org/10.1037/0735-7044.119.4.1125
McCann KE, Sinkiewicz DM, Norvelle A, Huhman KL (2017) De novo assembly, annotation, and characterization of the whole brain transcriptome of male and female Syrian hamsters. Sci Rep 7:40472. https://doi.org/10.1038/srep40472
De Vries GJ (2004) Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology 145(3):1063–1068. https://doi.org/10.1210/en.2003-1504
de Vries GJ, Forger NG (2015) Sex differences in the brain: a whole body perspective. Biol Sex Differ 6:15. https://doi.org/10.1186/s13293-015-0032-z
Marrocco J, Petty GH, Rios MB, Gray JD, Kogan JF, Waters EM, Schmidt EF, Lee FS et al (2017) A sexually dimorphic pre-stressed translational signature in CA3 pyramidal neurons of BDNF Val66Met mice. Nat Commun 8(1):808. https://doi.org/10.1038/s41467-017-01014-4
Labonte B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, Scarpa JR, Moy G et al (2017) Sex-specific transcriptional signatures in human depression. Nat Med 23(9):1102–1111. https://doi.org/10.1038/nm.4386
Mead AN, Stephens DN (2003) Involvement of AMPA receptor GluR2 subunits in stimulus-reward learning: evidence from glutamate receptor gria2 knock-out mice. J Neurosci 23(29):9500–9507
Gray AL, Hyde TM, Deep-Soboslay A, Kleinman JE, Sodhi MS (2015) Sex differences in glutamate receptor gene expression in major depression and suicide. Mol Psychiatry 20(9):1057–1068. https://doi.org/10.1038/mp.2015.91
Gulinello M, Gong QH, Smith SS (2002) Progesterone withdrawal increases the alpha4 subunit of the GABA(A) receptor in male rats in association with anxiety and altered pharmacology—a comparison with female rats. Neuropharmacology 43(4):701–714
Gulinello M, Orman R, Smith SS (2003) Sex differences in anxiety, sensorimotor gating and expression of the alpha4 subunit of the GABAA receptor in the amygdala after progesterone withdrawal. Eur J Neurosci 17(3):641–648
Tripp A, Oh H, Guilloux JP, Martinowich K, Lewis DA, Sibille E (2012) Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry 169(11):1194–1202. https://doi.org/10.1176/appi.ajp.2012.12020248
Ortiz JB, Taylor SB, Hoffman AN, Campbell AN, Lucas LR, Conrad CD (2015) Sex-specific impairment and recovery of spatial learning following the end of chronic unpredictable restraint stress: potential relevance of limbic GAD. Behav Brain Res 282:176–184. https://doi.org/10.1016/j.bbr.2014.12.051
Feldblum S, Erlander MG, Tobin AJ (1993) Different distributions of GAD65 and GAD67 mRNAS suggest that the two glutamate decarboxylases play distinctive functional roles. J Neurosci Res 34(6):689–706. https://doi.org/10.1002/jnr.490340612
Bowers G, Cullinan WE, Herman JP (1998) Region-specific regulation of glutamic acid decarboxylase (GAD) mRNA expression in central stress circuits. J Neurosci 18(15):5938–5947
Morrison KE, Swallows CL, Cooper MA (2011) Effects of dominance status on conditioned defeat and expression of 5-HT1A and 5-HT2A receptors. Physiol Behav 104(2):283–290. https://doi.org/10.1016/j.physbeh.2011.02.033
Bernstein HG, Dobrowolny H, Schott BH, Gorny X, Becker V, Steiner J, Seidenbecher CI, Bogerts B (2013) Increased density of AKAP5-expressing neurons in the anterior cingulate cortex of subjects with bipolar disorder. J Psychiatr Res 47(6):699–705. https://doi.org/10.1016/j.jpsychires.2012.12.020
Qi XR, Zhao J, Liu J, Fang H, Swaab DF, Zhou JN (2015) Abnormal retinoid and TrkB signaling in the prefrontal cortex in mood disorders. Cereb Cortex 25(1):75–83. https://doi.org/10.1093/cercor/bht203
Zhou R, Niwa S, Guillaud L, Tong Y, Hirokawa N (2013) A molecular motor, KIF13A, controls anxiety by transporting the serotonin type 1A receptor. Cell Rep 3(2):509–519. https://doi.org/10.1016/j.celrep.2013.01.014
Soleimani L, Roder JC, Dennis JW, Lipina T (2008) Beta N-acetylglucosaminyltransferase V (Mgat5) deficiency reduces the depression-like phenotype in mice. Genes Brain Behav 7(3):334–343. https://doi.org/10.1111/j.1601-183X.2007.00358.x
Wingo AP, Almli LM, Stevens JJ, Klengel T, Uddin M, Li Y, Bustamante AC, Lori A et al (2015) DICER1 and microRNA regulation in post-traumatic stress disorder with comorbid depression. Nat Commun 6:10106. https://doi.org/10.1038/ncomms10106
Yamamoto A, Uchiyama K, Nara T, Nishimura N, Hayasaka M, Hanaoka K, Yamamoto T (2014) Structural abnormalities of corpus callosum and cortical axonal tracts accompanied by decreased anxiety-like behavior and lowered sociability in spock3-mutant mice. Dev Neurosci 36(5):381–395. https://doi.org/10.1159/000363101
Dias C, Feng J, Sun H, Shao NY, Mazei-Robison MS, Damez-Werno D, Scobie K, Bagot R et al (2014) Beta-catenin mediates stress resilience through Dicer1/microRNA regulation. Nature 516(7529):51–55. https://doi.org/10.1038/nature13976
Lee JY, Kwak M, Lee PC (2015) Impairment of social behavior and communication in mice lacking the Uba6-dependent ubiquitin activation system. Behav Brain Res 281:78–85. https://doi.org/10.1016/j.bbr.2014.12.019
Gray CL, Norvelle A, Larkin T, Huhman KL (2015) Dopamine in the nucleus accumbens modulates the memory of social defeat in Syrian hamsters (Mesocricetus auratus). Behav Brain Res 286:22–28. https://doi.org/10.1016/j.bbr.2015.02.030
Ross AP, Norvelle A, Choi DC, Walton JC, Albers HE, Huhman KL (2017) Social housing and social isolation: Impact on stress indices and energy balance in male and female Syrian hamsters (Mesocricetus auratus). Physiol Behav 177:264–269. https://doi.org/10.1016/j.physbeh.2017.05.015
Potegal M, Huhman K, Moore T, Meyerhoff J (1993) Conditioned defeat in the Syrian golden hamster (Mesocricetus auratus). Behav Neural Biol 60(2):93–102
Albers HE, Huhman KL, Meisel RL (2002) Hormonal basis of social conflict and communication. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Moss RL, Rubin RR (eds) Hormones, brain and behavior. Academic Press, San Diego, p 393–433
McCann KE, Bicknese CN, Norvelle A, Huhman KL (2014) Effects of inescapable versus escapable social stress in Syrian hamsters: the importance of stressor duration versus escapability. Physiol Behav 129:25–29. https://doi.org/10.1016/j.physbeh.2014.02.039
McCann KE, Huhman KL (2012) The effect of escapable versus inescapable social defeat on conditioned defeat and social recognition in Syrian hamsters. Physiol Behav 105(2):493–497. https://doi.org/10.1016/j.physbeh.2011.09.009
Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, Magida J, Brancato A et al (2015) Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J Neurosci 35(50):16362–16376. https://doi.org/10.1523/jneurosci.1392-15.2015
Bagot RC, Cates HM, Purushothaman I, Vialou V, Heller EA, Yieh L, LaBonte B, Pena CJ et al (2017) Ketamine and imipramine reverse transcriptional signatures of susceptibility and induce resilience-specific gene expression profiles. Biol Psychiatry 81(4):285–295. https://doi.org/10.1016/j.biopsych.2016.06.012
Feng J, Wilkinson M, Liu X, Purushothaman I, Ferguson D, Vialou V, Maze I, Shao N et al (2014) Chronic cocaine-regulated epigenomic changes in mouse nucleus accumbens. Genome Biol 15(4):R65. https://doi.org/10.1186/gb-2014-15-4-r65
Konczal M, Koteja P, Stuglik MT, Radwan J, Babik W (2014) Accuracy of allele frequency estimation using pooled RNA-Seq. Mol Ecol Resour 14(2):381–392. https://doi.org/10.1111/1755-0998.12186
Sarajlic S, Edirisinghe N, Lukinov Y, Walters M, Davis B, Faroux G (2016) Orion: discovery environmenT for HPC research and bridging XSEDE resources. Paper presented at the Proceedings of the XSEDE16 Conference on Diversity, Big Data, and Science at Scale, Miami, USA,
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L et al (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29(7):644–652. https://doi.org/10.1038/nbt.1883
Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, Couger MB, Eccles D et al (2013) De novo transcript sequence reconstruction from RNA-seq using the trinity platform for reference generation and analysis. Nat Protoc 8(8):1494–1512. https://doi.org/10.1038/nprot.2013.084
Feng NY, Fergus DJ, Bass AH (2015) Neural transcriptome reveals molecular mechanisms for temporal control of vocalization across multiple timescales. BMC Genomics 16:408. https://doi.org/10.1186/s12864-015-1577-2
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410. https://doi.org/10.1016/s0022-2836(05)80360-2
UniProt C (2015) UniProt: a hub for protein information. Nucleic Acids Res 43(Database issue):D204–D212. https://doi.org/10.1093/nar/gku989
Li B, Dewey CN (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12(1):1–16. https://doi.org/10.1186/1471-2105-12-323
Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26(1):139–140. https://doi.org/10.1093/bioinformatics/btp616
Plaisier SB, Taschereau R, Wong JA, Graeber TG (2010) Rank-rank hypergeometric overlap: identification of statistically significant overlap between gene-expression signatures. Nucleic Acids Res 38(17):e169. https://doi.org/10.1093/nar/gkq636
Mi H, Muruganujan A, Casagrande JT, Thomas PD (2013) Large-scale gene function analysis with the PANTHER classification system. Nat Protoc 8(8):1551–1566. https://doi.org/10.1038/nprot.2013.092
Mi H, Poudel S, Muruganujan A, Casagrande JT, Thomas PD (2016) PANTHER version 10: expanded protein families and functions, and analysis tools. Nucleic Acids Res 44(D1):D336–D342. https://doi.org/10.1093/nar/gkv1194
Mi H, Thomas P (2009) PANTHER pathway: an ontology-based pathway database coupled with data analysis tools. Methods Mol Biol 563:123–140. https://doi.org/10.1007/978-1-60761-175-2_7
Young MD, Wakefield MJ, Smyth GK, Oshlack A (2010) Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol 11(2):R14. https://doi.org/10.1186/gb-2010-11-2-r14
Langfelder P, Zhang B, Horvath S (2008) Defining clusters from a hierarchical cluster tree: the dynamic tree cut package for R. Bioinformatics 24:719–720. https://doi.org/10.1093/bioinformatics/btm563
Langfelder P, Horvath S (2008) WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9(1):1–13. https://doi.org/10.1186/1471-2105-9-559
Acknowledgements
The authors gratefully acknowledge Dr. James C. Walton, Alisa Norvelle, M.S., and Georgia State University’s Research Solutions and High Performance Computing Group for their expert assistance. The authors would also like to thank Reed A. Gilbert, Atit A. Patel, and Dr. Brittany M. Thompson for their technical assistance. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Georgia State University.
Funding
This project was supported by NIH RO1MH062044 to KLH, Georgia State University Brains and Behavior Seed Grant to KLH, Georgia State University Dissertation Grant to KEM, Brains and Behavior Fellowships to KEM, DMS, and AMR, Honeycutt Fellowships to KEM and AMR, and a Next Generation Postdoctoral Fellowship from the Center for Behavioral Neuroscience to LQB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures and protocols were approved by the Georgia State University Institutional Animal Care and Use Committee and are in accordance with the standards outlined in the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Conflict of Interest
The authors declare they have no conflict of interest.
Electronic Supplementary Material
ESM 1
(PDF 1818 kb)
Rights and permissions
About this article
Cite this article
McCann, K.E., Sinkiewicz, D.M., Rosenhauer, A.M. et al. Transcriptomic Analysis Reveals Sex-Dependent Expression Patterns in the Basolateral Amygdala of Dominant and Subordinate Animals After Acute Social Conflict. Mol Neurobiol 56, 3768–3779 (2019). https://doi.org/10.1007/s12035-018-1339-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1339-7