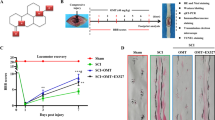
Abstract
Spinal cord injury (SCI) is a severe neurological disease with few efficacious drugs. Autophagy is a cellular process to confront with stress after SCI and considered to be a therapeutic target of SCI. In this study, we investigated the therapeutic effect of metformin on functional recovery after SCI and its underlying mechanism of autophagy regulation. Using a rat model of traumatic SCI, we found improved function recovery which was paralleled by a reduction of apoptosis after metformin treatment. We further examined autophagy via detecting autophagosomes by transmission electron microscopy and immunofluorescence, as well as autophagy markers by western blot in each groups. The results showed that the number of autophagosomes and expression of autophagy markers such as LC3 and beclin1 were increased in SCI group, while autophagy substrate protein p62 as well as ubiquitinated proteins were found to accumulate in SCI group, indicating an impaired autophagy flux in SCI. But, metformin treatment attenuated the accumulation of p62 and ubiquitinated proteins, suggesting a stimulative effect of autophagy flux by metformin. Blockage of autophagy flux by chloroquine partially abolished the apoptosis inhibition and functional recovery effect of metformin on SCI, which suggested that the protective effect of metformin on SCI was through autophagy flux stimulation. Activation of AMPK as well as inhibition of its downstream mTOR signaling were detected under metformin treatment in vivo and in vitro; inhibition of AMPK signaling by compound C suppressed autophagy flux induced by metformin in vitro, indicating that AMPK signaling was involved in the effect of metformin on autophagy flux regulation. Together, these results illustrated that metformin improved functional recovery effect through autophagy flux stimulation and implied metformin to be a potential drug for SCI therapy.









Similar content being viewed by others
References
Zhu SP, Wang ZG, Zhao YZ, Wu J, Shi HX, Ye LB, Wu FZ, Cheng Y, Zhang HY, He S, Wei X, Fu XB, Li XK, Xu HZ, Xiao J (2015) Gelatin nanostructured lipid carriers incorporating nerve growth factor inhibit endoplasmic reticulum stress-induced apoptosis and improve recovery in spinal cord injury. Molecular neurobiology. doi:10.1007/s12035-015-9372-2
Warden P, Bamber NI, Li H, Esposito A, Ahmad KA, Hsu CY, Xu XM (2001) Delayed glial cell death following wallerian degeneration in white matter tracts after spinal cord dorsal column cordotomy in adult rats. Exp Neurol 168(2):213–224. doi:10.1006/exnr.2000.7622
Bethea JR, Dietrich WD (2002) Targeting the host inflammatory response in traumatic spinal cord injury. Curr Opin Neurol 15(3):355–360
Choi DC, Lee JY, Moon YJ, Kim SW, Oh TH, Yune TY (2010) Acupuncture-mediated inhibition of inflammation facilitates significant functional recovery after spinal cord injury. Neurobiol Dis 39(3):272–282. doi:10.1016/j.nbd.2010.04.003
Wu KL, Chan SH, Chao YM, Chan JY (2003) Expression of pro-inflammatory cytokine and caspase genes promotes neuronal apoptosis in pontine reticular formation after spinal cord transection. Neurobiol Dis 14(1):19–31
Casha S, Yu WR, Fehlings MG (2005) FAS deficiency reduces apoptosis, spares axons and improves function after spinal cord injury. Exp Neurol 196(2):390–400. doi:10.1016/j.expneurol.2005.08.020
Cavallucci V, D’Amelio M (2011) Matter of life and death: the pharmacological approaches targeting apoptosis in brain diseases. Curr Pharm Des 17(3):215–229
Li HT, Zhao XZ, Zhang XR, Li G, Jia ZQ, Sun P, Wang JQ, Fan ZK, Lv G (2015) Exendin-4 enhances motor function recovery via promotion of autophagy and inhibition of neuronal apoptosis after spinal cord injury in rats. Molecular neurobiology. doi:10.1007/s12035-015-9327-7
Yang Z, Klionsky DJ (2010) Eaten alive: a history of macroautophagy. Nat Cell Biol 12(9):814–822. doi:10.1038/ncb0910-814
Levine B, Klionsky DJ (2004) Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6(4):463–477
Mizushima N (2011) Autophagy in protein and organelle turnover. Cold Spring Harb Symp Quant Biol 76:397–402. doi:10.1101/sqb.2011.76.011023
Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M et al (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8(4):445–544
Bove J, Martinez-Vicente M, Vila M (2011) Fighting neurodegeneration with rapamycin: mechanistic insights. Nat Rev Neurosci 12(8):437–452. doi:10.1038/nrn3068
Nixon RA (2006) Autophagy in neurodegenerative disease: friend, foe or turncoat? Trends Neurosci 29(9):528–535. doi:10.1016/j.tins.2006.07.003
Kanno H, Ozawa H, Sekiguchi A, Itoi E (2009) Spinal cord injury induces upregulation of Beclin 1 and promotes autophagic cell death. Neurobiol Dis 33(2):143–148. doi:10.1016/j.nbd.2008.09.009
Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Itoi E (2011) Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine (Phila Pa 1976) 36(22):E1427–1434. doi:10.1097/BRS.0b013e3182028c3a
Chen HC, Fong TH, Lee AW, Chiu WT (2012) Autophagy is activated in injured neurons and inhibited by methylprednisolone after experimental spinal cord injury. Spine (Phila Pa 1976) 37(6):470–475. doi:10.1097/BRS.0b013e318221e859
Sekiguchi A, Kanno H, Ozawa H, Yamaya S, Itoi E (2012) Rapamycin promotes autophagy and reduces neural tissue damage and locomotor impairment after spinal cord injury in mice. J Neurotrauma 29(5):946–956. doi:10.1089/neu.2011.1919
Kim J, Kim TY, Cho KS, Kim HN, Koh JY (2013) Autophagy activation and neuroprotection by progesterone in the G93A-SOD1 transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 59:80–85. doi:10.1016/j.nbd.2013.07.011
Zhang HY, Wang ZG, Wu FZ, Kong XX, Yang J, Lin BB, Zhu SP, Lin L et al (2013) Regulation of autophagy and ubiquitinated protein accumulation by bFGF promotes functional recovery and neural protection in a rat model of spinal cord injury. Mol Neurobiol 48(3):452–464. doi:10.1007/s12035-013-8432-8
Tang P, Hou H, Zhang L, Lan X, Mao Z, Liu D, He C, Du H et al (2014) Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats. Mol Neurobiol 49(1):276–287. doi:10.1007/s12035-013-8518-3
Liu S, Sarkar C, Dinizo M, Faden AI, Koh EY, Lipinski MM, Wu J (2015) Disrupted autophagy after spinal cord injury is associated with ER stress and neuronal cell death. Cell Death Dis 6:e1582. doi:10.1038/cddis.2014.527
Shin JY, Park HJ, Kim HN, Oh SH, Bae JS, Ha HJ, Lee PH (2014) Mesenchymal stem cells enhance autophagy and increase beta-amyloid clearance in Alzheimer disease models. Autophagy 10(1):32–44. doi:10.4161/auto.26508
Hayashi S, Sato N, Yamamoto A, Ikegame Y, Nakashima S, Ogihara T, Morishita R (2009) Alzheimer disease-associated peptide, amyloid beta40, inhibits vascular regeneration with induction of endothelial autophagy. Arterioscler Thromb Vasc Biol 29(11):1909–1915. doi:10.1161/ATVBAHA.109.188516
Gan-Or Z, Dion PA, Rouleau GA (2015) Genetic perspective on the role of the autophagy-lysosome pathway in Parkinson disease. Autophagy 11(9):1443–1457. doi:10.1080/15548627.2015.1067364
Yamamoto A, Cremona ML, Rothman JE (2006) Autophagy-mediated clearance of huntingtin aggregates triggered by the insulin-signaling pathway. J Cell Biol 172(5):719–731. doi:10.1083/jcb.200510065
Zhang X, Chen S, Song L, Tang Y, Shen Y, Jia L, Le W (2014) MTOR-independent, autophagic enhancer trehalose prolongs motor neuron survival and ameliorates the autophagic flux defect in a mouse model of amyotrophic lateral sclerosis. Autophagy 10(4):588–602. doi:10.4161/auto.27710
Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ (2008) Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes 57(3):696–705. doi:10.2337/db07-1098
Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, Prigaro BJ, Wood JL et al (2014) Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510(7506):542–546. doi:10.1038/nature13270
Patil SP, Jain PD, Ghumatkar PJ, Tambe R, Sathaye S (2014) Neuroprotective effect of metformin in MPTP-induced Parkinson’s disease in mice. Neuroscience 277:747–754. doi:10.1016/j.neuroscience.2014.07.046
Vazquez-Manrique RP, Farina F, Cambon K, Sequedo MD, Parker AJ, Millan JM, Weiss A, Deglon N, Neri C (2015) AMPK activation protects from neuronal dysfunction and vulnerability across nematode, cellular and mouse models of Huntington’s disease. Human molecular genetics. doi:10.1093/hmg/ddv513
Liu Y, Tang G, Zhang Z, Wang Y, Yang GY (2014) Metformin promotes focal angiogenesis and neurogenesis in mice following middle cerebral artery occlusion. Neurosci Lett 579:46–51. doi:10.1016/j.neulet.2014.07.006
Duca FA, Cote CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, Lam TK (2015) Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat Med 21(5):506–511. doi:10.1038/nm.3787
Perrin FE, Boniface G, Serguera C, Lonjon N, Serre A, Prieto M, Mallet J, Privat A (2010) Grafted human embryonic progenitors expressing neurogenin-2 stimulate axonal sprouting and improve motor recovery after severe spinal cord injury. PLoS One 5(12):e15914. doi:10.1371/journal.pone.0015914
Sugawara T, Lewen A, Gasche Y, Yu F, Chan PH (2002) Overexpression of SOD1 protects vulnerable motor neurons after spinal cord injury by attenuating mitochondrial cytochrome c release. FASEB J 16(14):1997–1999. doi:10.1096/fj.02-0251fje
Mitsuhara T, Takeda M, Yamaguchi S, Manabe T, Matsumoto M, Kawahara Y, Yuge L, Kurisu K (2013) Simulated microgravity facilitates cell migration and neuroprotection after bone marrow stromal cell transplantation in spinal cord injury. Stem Cell Res Ther 4(2):35. doi:10.1186/scrt184
Dubreuil CI, Winton MJ, McKerracher L (2003) Rho activation patterns after spinal cord injury and the role of activated Rho in apoptosis in the central nervous system. J Cell Biol 162(2):233–243. doi:10.1083/jcb.200301080
Lamberts RR, Onderwater G, Hamdani N, Vreden MJ, Steenhuisen J, Eringa EC, Loer SA, Stienen GJ et al (2009) Reactive oxygen species-induced stimulation of 5’AMP-activated protein kinase mediates sevoflurane-induced cardioprotection. Circulation 120(11 Suppl):S10–15. doi:10.1161/CIRCULATIONAHA.108.828426
Abdelsaid M, Prakash R, Li W, Coucha M, Hafez S, Johnson MH, Fagan SC, Ergul A (2015) Metformin treatment in the period after stroke prevents nitrative stress and restores angiogenic signaling in the brain in diabetes. Diabetes 64(5):1804–1817. doi:10.2337/db14-1423
Ashabi G, Khodagholi F, Khalaj L, Goudarzvand M, Nasiri M (2014) Activation of AMP-activated protein kinase by metformin protects against global cerebral ischemia in male rats: interference of AMPK/PGC-1alpha pathway. Metab Brain Dis 29(1):47–58. doi:10.1007/s11011-013-9475-2
Zheng B, Ye L, Zhou Y, Zhu S, Wang Q, Shi H, Chen D, Wei X, Wang Z, Li X, Xiao J, Xu H, Zhang H (2016) Epidermal growth factor attenuates blood-spinal cord barrier disruption via PI3K/Akt/Rac1 pathway after acute spinal cord injury. Journal of cellular and molecular medicine. doi:10.1111/jcmm.12761
Zhou Y, Zheng B, Ye L, Zhang H, Zhu S, Zheng X, Xia Q, He Z, Wang Q, Xiao J, Xu H (2015) Retinoic acid prevents disruption of blood-spinal cord barrier by inducing autophagic flux after spinal cord injury. Neurochemical research. doi:10.1007/s11064-015-1756-1
Hong JY, Lee SH, Lee SC, Kim JW, Kim KP, Kim SM, Tapia N, Lim KT et al (2014) Therapeutic potential of induced neural stem cells for spinal cord injury. J Biol Chem 289(47):32512–32525. doi:10.1074/jbc.M114.588871
El-Mir MY, Detaille D, R-Villanueva G, Delgado-Esteban M, Guigas B, Attia S, Fontaine E, Almeida A et al (2008) Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons. J Mol Neurosci 34(1):77–87. doi:10.1007/s12031-007-9002-1
Sakurai M, Nagata T, Abe K, Horinouchi T, Itoyama Y, Tabayashi K (2003) Survival and death-promoting events after transient spinal cord ischemia in rabbits: induction of Akt and caspase3 in motor neurons. J Thorac Cardiovasc Surg 125(2):370–377. doi:10.1067/mtc.2003.112
Zhang J, Cui Z, Feng G, Bao G, Xu G, Sun Y, Wang L, Chen J et al (2015) RBM5 and p53 expression after rat spinal cord injury: implications for neuronal apoptosis. Int J Biochem Cell Biol 60:43–52. doi:10.1016/j.biocel.2014.12.020
Cahova M, Palenickova E, Dankova H, Sticova E, Burian M, Drahota Z, Cervinkova Z, Kucera O et al (2015) Metformin prevents ischemia reperfusion-induced oxidative stress in the fatty liver by attenuation of reactive oxygen species formation. Am J Physiol Gastrointest Liver Physiol 309(2):G100–111. doi:10.1152/ajpgi.00329.2014
Elmadhun NY, Sabe AA, Lassaletta AD, Chu LM, Sellke FW (2014) Metformin mitigates apoptosis in ischemic myocardium. J Surg Res 192(1):50–58. doi:10.1016/j.jss.2014.05.005
Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, Zhang QQ, Gao L et al (2014) Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol 171(13):3146–3157. doi:10.1111/bph.12655
Snigdha S, Smith ED, Prieto GA, Cotman CW (2012) Caspase-3 activation as a bifurcation point between plasticity and cell death. Neurosci Bull 28(1):14–24. doi:10.1007/s12264-012-1057-5
Adams JM, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281(5381):1322–1326
Rudel T (1999) Caspase inhibitors in prevention of apoptosis. Herz 24(3):236–241
Ullah I, Ullah N, Naseer MI, Lee HY, Kim MO (2012) Neuroprotection with metformin and thymoquinone against ethanol-induced apoptotic neurodegeneration in prenatal rat cortical neurons. BMC Neurosci 13:11. doi:10.1186/1471-2202-13-11
Ding F, Shao ZW, Xiong LM (2013) Cell death in intervertebral disc degeneration. Apoptosis 18(7):777–785. doi:10.1007/s10495-013-0839-1
Kanno H, Ozawa H, Sekiguchi A, Itoi E (2009) The role of autophagy in spinal cord injury. Autophagy 5(3):390–392
Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Tateda S, Yahata K, Itoi E (2012) The role of mTOR signaling pathway in spinal cord injury. Cell Cycle 11(17):3175–3179. doi:10.4161/cc.21262
Lipinski MM, Wu J, Faden AI, Sarkar C (2015) Function and mechanisms of autophagy in brain and spinal cord trauma. Antioxid Redox Signal 23(6):565–577. doi:10.1089/ars.2015.6306
Cordaro M, Paterniti I, Siracusa R, Impellizzeri D, Esposito E, Cuzzocrea S (2016) KU0063794, a dual mTORC1 and mTORC2 inhibitor, reduces neural tissue damage and locomotor impairment after spinal cord injury in mice. Molecular neurobiology. doi:10.1007/s12035-016-9827-0
Rigacci S, Miceli C, Nediani C, Berti A, Cascella R, Pantano D, Nardiello P, Luccarini I, Casamenti F, Stefani M (2015) Oleuropein aglycone induces autophagy via the AMPK/mTOR signalling pathway: a mechanistic insight. Oncotarget 6(34):35344–35357, doi:10.18632/oncotarget.6119
Jia Y, Wang H, Wang Q, Ding H, Wu H, Pan H (2015) Silencing Nrf2 impairs glioma cell proliferation via AMPK-activated mTOR inhibition. Biochemical and biophysical research communications. doi:10.1016/j.bbrc.2015.12.034
Lee JH, Jeong JK, Park SY (2014) Sulforaphane-induced autophagy flux prevents prion protein-mediated neurotoxicity through AMPK pathway. Neuroscience 278:31–39. doi:10.1016/j.neuroscience.2014.07.072
Hou YS, Guan JJ, Xu HD, Wu F, Sheng R, Qin ZH (2015) Sestrin2 protects dopaminergic cells against rotenone toxicity through AMPK-dependent autophagy activation. Mol Cell Biol 35(16):2740–2751. doi:10.1128/MCB.00285-15
Acknowledgments
This study was supported by National Natural Science Foundation of China (81401871, 81401162, 81572227, 81501907, and 81371988), Zhejiang Provincial Natural Science Foundation of China (LY15H060008, LY14H170002, and Y2110466), Zhejiang Medical Science Foundation (2013KYA127 and 2013KYB177), and Wenzhou Science and Technology Bereau Foundation (S20100048 and Y20100357)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, D., Xuan, J., Zheng, Bb. et al. Metformin Improves Functional Recovery After Spinal Cord Injury via Autophagy Flux Stimulation. Mol Neurobiol 54, 3327–3341 (2017). https://doi.org/10.1007/s12035-016-9895-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9895-1