Abstract
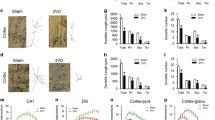
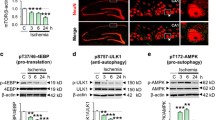
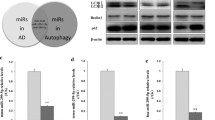
Chronic brain hypoperfusion (CBH) induces the accumulation of abnormal cellular proteins, accompanied by cognitive decline, and the autophagic-lysosomal system is abnormal in dementia. Whether CBH accounts for autophagic-lysosomal neuropathology remains unknown. Here, we show that CBH significantly increased the number of autophagic vacuoles (AVs) with high LC3-II levels, but decreased SQSTM1 and cathepsin D levels in the hippocampi of rats following bilateral common carotid artery occlusion (2VO) for 2 weeks. Further studies showed that microRNA-27a (Mir27a) was upregulated at 2 weeks compared with the sham group. Additionally, LAMP-2 proteins were downregulated by Mir27a overexpression, upregulated by Mir27a inhibition, and unchanged by binding-site mutations or miR-masks, indicating that lamp-2 is the target of Mir27a. Knockdown of endogenous Mir27a prevented the reduction of LAMP-2 protein expression as well as the accumulation of AVs in the hippocampi of 2VO rats. Overexpression of Mir27a induced, while the knockdown of Mir27a reduced, the accumulation of AVs and the LC3-II level in cultured neonatal rat neurons. The results revealed that CBH in rats at 2 weeks could induce inefficient lysosomal clearance, which is regulated by the Mir27a-mediated downregulation of LAMP-2 protein expression. These findings provide an insight into a novel molecular mechanism of autophagy at the miRNA level.








Similar content being viewed by others
Abbreviations
- miRNA:
-
MicroRNA
- Mir27a:
-
MicroRNA-27a
- AMO-27a:
-
2′-O-Methyl antisense oligoribonucleotides to miR-27a
- NC:
-
Scramble negative control
- ODN:
-
miRNA-masking antisense oligodeoxynucleotides (miR-masks)
- LC3:
-
Microtubule-associated protein 1 light chain 3
- LAMP-2:
-
Lysosomal-associated membrane protein-2
- SQSTM1/P62:
-
Sequestosome1
- MTOR:
-
Mechanistic target of rapamycin
- p70S6k:
-
Ribosomal protein S6 kinase 70kDa
- AVs:
-
Autophagic vaculoses
- AL:
-
Autolysosome
- 3MA:
-
3-Methyladenine
- Rap:
-
Rapamycin
- 3′UTR:
-
3′-Untranslated region
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- 2VO:
-
Bilateral common carotid artery occlusion
References
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075. doi:10.1038/nature06639
Nixon RA (2006) Autophagy in neurodegenerative disease: friend, foe or turncoat? Trends Neurosci 29:528–535
Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441:885–889
Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D (2004) Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305:1292–1295
Kegel KB, Kim M, Sapp E, McIntyre C, Castano JG, Aronin N, DiFiglia M (2000) Huntingtin expression stimulates endosomal-lysosomal activity, endosome tubulation, and autophagy. J Neurosci 20:7268–7278
Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, Mohan PS, Mercken M, Farmery MR, Tjernberg LO, Jiang Y, Duff K, Uchiyama Y, Naslund J, Mathews PM, Cataldo AM, Nixon RA (2005) Macroautophagy—a novel Beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. J Cell Biol 171:87–98
Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, Uchiyama Y, Westaway D, Cuervo AM, Nixon RA (2010) Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 141:1146–1158. doi:10.1016/j.cell.2010.05.008
Funderburk SF, Marcellino BK, Yue Z (2010) Cell “self-eating” (autophagy) mechanism in Alzheimer’s disease. Mt Sinai J Med 77:59–68. doi:10.1002/msj.20161
Nixon RA, Yang DS (2011) Autophagy failure in Alzheimer’s disease—locating the primary defect. Neurobiol Dis 43:38–45. doi:10.1016/j.nbd.2011.01.021
Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA (2008) Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci 28:6926–6937. doi:10.1523/JNEUROSCI.0800-08.2008
Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MM (2005) Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol 57:789–794
Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S (2011) Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42:2672–2713. doi:10.1161/STROKEAHA.111.634279
Farkas E, Luiten PG, Bari F (2007) Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev 54:162–180
Ai J, Sun LH, Che H, Zhang R, Zhang TZ, Wu WC, Su XL, Chen X, Yang G, Li K, Wang N, Ban T, Bao YN, Guo F, Niu HF, Zhu YL, Zhu XY, Zhao SG, Yang BF (2013) MicroRNA-195 protects against dementia induced by chronic brain hypoperfusion via its anti-amyloidogenic effect in rats. J Neurosci 33:3989–4001. doi:10.1523/JNEUROSCI.1997-12.2013
Kitaguchi H, Tomimoto H, Ihara M, Shibata M, Uemura K, Kalaria RN, Kihara T, Asada-Utsugi M, Kinoshita A, Takahashi R (2009) Chronic cerebral hypoperfusion accelerates amyloid beta deposition in APPSwInd transgenic mice. Brain Res 1294:202–210. doi:10.1016/j.brainres.2009.07.078
Zhiyou C, Yong Y, Shanquan S, Jun Z, Liangguo H, Ling Y, Jieying L (2009) Upregulation of BACE1 and beta-amyloid protein mediated by chronic cerebral hypoperfusion contributes to cognitive impairment and pathogenesis of Alzheimer’s disease. Neurochem Res 34:1226–1235. doi:10.1007/s11064-008-9899-y
Sun LH, Ban T, Liu CD, Chen QX, Wang X, Yan ML, Hu XL, Su XL, Bao YN, Sun LL, Zhao LJ, Pei SC, Jiang XM, Zong DK, Ai J (2015) Activation of Cdk5/p25 and tau phosphorylation following chronic brain hypoperfusion in rats involves microRNA-195 down-regulation. J Neurochem 134:1139–1151. doi:10.1111/jnc.13212
Farkas E, Institoris A, Domoki F, Mihaly A, Bari F (2006) The effect of pre- and posttreatment with diazoxide on the early phase of chronic cerebral hypoperfusion in the rat. Brain Res 1087:168–174
Liu HX, Zhang JJ, Zheng P, Zhang Y (2005) Altered expression of MAP-2, GAP-43, and synaptophysin in the hippocampus of rats with chronic cerebral hypoperfusion correlates with cognitive impairment. Brain Res Mol Brain Res 139:169–177
Alvarez-Erviti L, Seow Y, Schapira AH, Rodriguez-Oroz MC, Obeso JA, Cooper JM (2013) Influence of microRNA deregulation on chaperone-mediated autophagy and alpha-synuclein pathology in Parkinson’s disease. Cell Death Dis 4:e545. doi:10.1038/cddis.2013.73
Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S (2007) The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res 67:11001–11011
Tang W, Zhu J, Su S, Wu W, Liu Q, Su F, Yu F (2012) MiR-27 as a prognostic marker for breast cancer progression and patient survival. PLoS One 7:e51702. doi:10.1371/journal.pone.0051702
Shirasaki T, Honda M, Shimakami T, Horii R, Yamashita T, Sakai Y, Sakai A, Okada H, Watanabe R, Murakami S, Yi M, Lemon SM, Kaneko S (2013) MicroRNA-27a regulates lipid metabolism and inhibits hepatitis C virus replication in human hepatoma cells. J Virol 87:5270–5286. doi:10.1128/JVI.03022-12
Yeh CH, Chen TP, Wang YC, Lin YM, Fang SW (2012) MicroRNA-27a regulates cardiomyocytic apoptosis during cardioplegia-induced cardiac arrest by targeting interleukin 10-related pathways. Shock 38:607–614. doi:10.1097/SHK.0b013e318271f944
Kulshreshtha R, Davuluri RV, Calin GA, Ivan M (2008) A microRNA component of the hypoxic response. Cell Death Differ 15:667–671. doi:10.1038/sj.cdd.4402310
Chen Q, Xu J, Li L, Li H, Mao S, Zhang F, Zen K, Zhang CY, Zhang Q (2014) MicroRNA-23a/b and microRNA-27a/b suppress Apaf-1 protein and alleviate hypoxia-induced neuronal apoptosis. Cell Death Dis 5:e1132. doi:10.1038/cddis.2014.92
Kang BY, Park KK, Green DE, Bijli KM, Searles CD, Sutliff RL, Hart CM (2013) Hypoxia mediates mutual repression between microRNA-27a and PPARgamma in the pulmonary vasculature. PLoS One 8:e79503. doi:10.1371/journal.pone.0079503
Wu X, Bhayani MK, Dodge CT, Nicoloso MS, Chen Y, Yan X, Adachi M, Thomas L, Galer CE, Jiffar T, Pickering CR, Kupferman ME, Myers JN, Calin GA, Lai SY (2013) Coordinated targeting of the EGFR signaling axis by microRNA-27a*. Oncotarget 4:1388–1398
Kumaran D, Udayabanu M, Kumar M, Aneja R, Katyal A (2008) Involvement of angiotensin converting enzyme in cerebral hypoperfusion induced anterograde memory impairment and cholinergic dysfunction in rats. Neuroscience 155:626–639. doi:10.1016/j.neuroscience.2008.06.023
Carloni S, Girelli S, Scopa C, Buonocore G, Longini M, Balduini W (2010) Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxia-ischemia. Autophagy 6:366–377
Baek SH, Noh AR, Kim KA, Akram M, Shin YJ, Kim ES, Yu SW, Majid A, Bae ON (2014) Modulation of mitochondrial function and autophagy mediates carnosine neuroprotection against ischemic brain damage. Stroke 45:2438–2443. doi:10.1161/STROKEAHA.114.005183
Tanida I, Ueno T, Kominami E (2004) LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol 36:2503–2518
Barth S, Glick D, Macleod KF (2010) Autophagy: assays and artifacts. J Pathol 221:117–124. doi:10.1002/path.2694
Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171:603–614
Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282:24131–24145
Ma X, Liu H, Foyil SR, Godar RJ, Weinheimer CJ, Hill JA, Diwan A (2012) Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation 125:3170–3181. doi:10.1161/CIRCULATIONAHA.111.041814
Bohley P, Seglen PO (1992) Proteases and proteolysis in the lysosome. Experientia 48:151–157
Amritraj A, Wang Y, Revett TJ, Vergote D, Westaway D, Kar S (2013) Role of cathepsin D in U18666A-induced neuronal cell death: potential implication in Niemann-Pick type C disease pathogenesis. J Biol Chem 288:3136–3152. doi:10.1074/jbc.M112.412460
Eskelinen EL (2006) Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol Aspects Med 27:495–502
Saftig P, Beertsen W, Eskelinen EL (2008) LAMP-2: a control step for phagosome and autophagosome maturation. Autophagy 4:510–512
Saugstad JA (2010) MicroRNAs as effectors of brain function with roles in ischemia and injury, neuroprotection, and neurodegeneration. J Cereb Blood Flow Metab 30:1564–1576
Lee ST, Chu K, Jung KH, Yoon HJ, Jeon D, Kang KM, Park KH, Bae EK, Kim M, Lee SK, Roh JK (2010) MicroRNAs induced during ischemic preconditioning. Stroke 41:1646–1651. doi:10.1038/jcbfm.2010.101
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132:27–42. doi:10.1016/j.cell.2007.12.018
Seglen PO, Gordon PB (1982) 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci U S A 79:1889–1892
Massey AC, Follenzi A, Kiffin R, Zhang C, Cuervo AM (2008) Autophagy 4:442–456
Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, Mi N, Zhao Y, Liu Z, Wan F, Hailey DW, Oorschot V, Klumperman J, Baehrecke EH, Lenardo MJ (2010) Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature 465:942–946. doi:10.1038/nature09076
Chen Y, Yu L (2013) Autophagic lysosome reformation. Exp Cell Res 319:142–146. doi:10.1016/j.yexcr.2012.09.004
Adhami F, Schloemer A, Kuan CY (2007) The roles of autophagy in cerebral ischemia. Autophagy 3:42–44
Finn PF, Mesires NT, Vine M, Dice JF (2005) Effects of small molecules on chaperone-mediated autophagy. Autophagy 1:141–145
Althausen S, Mengesdorf T, Mies G, Olah L, Nairn AC, Proud CG, Paschen W (2001) Changes in the phosphorylation of initiation factor eIF-2alpha, elongation factor eEF-2 and p70 S6 kinase after transient focal cerebral ischaemia in mice. J Neurochem 78:779–787
Pastor MD, Garcia-Yebenes I, Fradejas N, Perez-Ortiz JM, Mora-Lee S, Tranque P, Moro MA, Pende M, Calvo S (2009) mTOR/S6 kinase pathway contributes to astrocyte survival during ischemia. J Biol Chem 284:22067–22078. doi:10.1074/jbc.M109.033100
Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K (2007) Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131:1149–1163
Kochl R, Hu XW, Chan EY, Tooze SA (2006) Microtubules facilitate autophagosome formation and fusion of autophagosomes with endosomes. Traffic 7:129–145
Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, Lullmann-Rauch R, Janssen PM, Blanz J, von Figura K, Saftig P (2000) Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature 406:902–906
Klionsky DJ, Abdalla FC, Abeliovich H et al (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8:445–544
Cuervo AM (2010) Chaperone-mediated autophagy: selectivity pays off. Trends Endocrinol Metab 21(3):142–150. doi:10.1016/j.tem.2009.10.003
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Natural Science Foundation of China (81070882, 81471115, 81271207 to J. A.) and the Creative Research Groups of the National Natural Science Foundation of China (81421063 to Y.B.F.).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Hui Che and Yan Yan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Che, H., Yan, Y., Kang, XH. et al. MicroRNA-27a Promotes Inefficient Lysosomal Clearance in the Hippocampi of Rats Following Chronic Brain Hypoperfusion. Mol Neurobiol 54, 2595–2610 (2017). https://doi.org/10.1007/s12035-016-9856-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9856-8