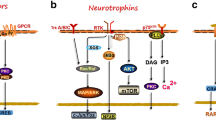
Abstract
Neurodegenerative diseases are still a challenge for researchers and clinicians due to its complexity. Traditional medicines usually do not provide sufficient protection against these diseases due to drug resistance and relapse. The discovery of the therapeutic potential of stem cells offers new opportunities for the treatment of incurable neurological diseases. Based on their biological properties, stem cells can differentiate into specific tissue type and maintain the cellular tissue/organ homeostasis in physiological and pathological conditions. Recently, it has been demonstrated that somatic cells of patients can be reprogrammed to a pluripotent state from which neural lineage cells can be derived. Potential strategies such as cell replacement therapy and gene transfer to the diseased or injured brain have opened a new line of therapeutic approach for a broad spectrum of human neurological diseases. Thus, stem cell replacement therapy for central and peripheral nervous system disorders aims at repopulating the affected neural tissue with new neurons. However, the limiting factors that have hampered the development of this promising therapeutic approach are the lack of suitable cell types for cell replacement therapy in patients suffering from neurological disorders. In this review, we have discussed the recent advances in stem cell replacement therapy with particular emphasis to neurological disorders.



Similar content being viewed by others
Abbreviations
- DA:
-
Dopaminergic
- ESCs:
-
Embryonic stem cells
- iPSCs:
-
Induced pluripotent stem cells
- iPS cells:
-
Induced pluripotent stem cells
- ADSCs:
-
Adipose-derived stromal cells
- BM-MSCs:
-
Bone marrow-derived mesenchymal stem cells
- NSCs:
-
Neural stem cells
- NGF:
-
Nerve growth factor
- BDNF:
-
Brain-derived growth factor
- Oct4:
-
Octamer-binding transcription factor 4
- Mbd3:
-
Methyl-CpG-binding domain protein 3
- NuRD:
-
Nucleosome remodeling and deacetylase
- Ascl1:
-
Achaete-scute complex homolog 1
- Brn2:
-
Brain-2
- Myt1l:
-
Myelin transcription factor 1-like
- NeuroD:
-
Neuronal differentiation
- OLIG2:
-
Oligodendrocyte lineage transcription factor 2
- Zic1:
-
Zinc finger protein of the cerebellum 1
- miR-9/9*:
-
Bifunctional microRNA strands 9
- miR-124:
-
MicroRNA 124
- Lmx1a:
-
LIM homeobox transcription factor 1 alpha
- Nurr1:
-
Nuclear receptor related 1
- Pitx3:
-
Paired-like homeodomain 3
- Foxa2:
-
Forkhead box A2
- EN1:
-
Engrailed homeobox 1
- Lhx3:
-
LIM homeobox 3
- Hb9:
-
Homeobox 9
- Isl1:
-
Islet 1 (ISL LIM homeobox 1)
- Ngn2:
-
Neurogenin 2
- Sox2:
-
Sex determining region Y box 2
- Klf4:
-
Krüppel-like factor 4
- c-Myc:
-
v-Myc avian myelocytomatosis viral oncogene homolog
- E47/Tcf3:
-
Transcription factor 3
- Aβ:
-
Amyloid-beta
- APP:
-
Amyloid precursor protein
- ASC:
-
Adult Stem Cells
- BM-MSC:
-
Bone marrow mesenchymal stem cell
- ChAT:
-
Choline-acetyltransferase
- EPI-NCSC:
-
Epidermal neural crest stem cell
- ES:
-
Embryonic stem cell
- NGFR:
-
Nerve growth factor receptor
- hNSC:
-
Human neural stem cell
- mNSC:
-
Murine neural stem cell
- UCB-MSC:
-
Umbilical cord blood mesenchymal stem cell
- LRRK2:
-
Leucine-rich repeat kinase 2
- PINK1:
-
PTEN-induced putative kinase 1
- Fbxo7:
-
F-Box only protein 7
- PSEN1:
-
Presenilin protein 1
- PSEN2:
-
Presenilin protein 2
- TREM2:
-
Triggering receptor expressed on myeloid cells 2
- APP:
-
Amyloid precursor protein
- SOD1:
-
Superoxide dismutase 1
- VAPB:
-
Vesicle-associated membrane protein-associated protein B/C
- TDP43:
-
TAR DNA-binding protein 43
- C9ORF72:
-
Chromosome 9 open reading frame 72
- FUS:
-
Fused in sarcoma
- ABCG2:
-
ATP-binding cassette sub-family G member 2
- CXCR4:
-
C-X-C chemokine receptor type 4
- FGF R4:
-
Fibroblast growth factor receptor 4
- Frizzled-9:
-
Frizzled class receptor 9
- Glut1:
-
Glucose transporter 1
- SSEA-1:
-
Stage-specific embryonic antigen 1
- BLBP/FABP7:
-
Brain lipid binding protein (also fatty acid binding protein 7)
- GLAST/SLC1A3:
-
Glutamate aspartate transporter or solute carrier family 1 (glial high-affinity glutamate transporter) member 3
- GFAP:
-
Glial fibrillary acidic protein
- S100B:
-
S100 calcium binding protein B
- PAX6:
-
Paired box protein 6
- TBR2:
-
T-box brain protein 2 or eomesodermin
- FGF:
-
Fibroblast growth factor
- Islet-1 and 2:
-
ISL LIM homeobox 1 and 2
- Lhx3:
-
LIM/homeobox protein 3
- Olig2:
-
Oligodendrocyte transcription factor
- MOG:
-
Myelin oligodendrocyte glycoprotein
- GalC:
-
Galactosylceramidase
- NeuN:
-
Feminizing locus on X-3 Fox-3 Rbfox3, or hexaribonucleotide binding protein-3
- NF-L:
-
Light neurofilament
- NF-M:
-
Medium neurofilament
- TH:
-
Tyrosine hydroxylase
- GAD:
-
Glutamic acid decarboxylase
- PSD-95:
-
Postsynaptic density protein 95
- VAMP:
-
Vesicle-associated membrane proteins
- EMT:
-
Mesenchymal to endothelial transition
References
Patel S, Singh V, Kumar A, Gupta YK, Singh MP (2006) Status of antioxidant defense system and expression of toxicant responsive genes in striatum of maneb- and paraquat-induced Parkinson’s disease phenotype in mouse: mechanism of neurodegeneration. Brain Res 1081(1):9–18. doi:10.1016/j.brainres.2006.01.060
Ferrante RJ, Kowall NW, Beal MF, Richardson EP Jr, Bird ED, Martin JB (1985) Selective sparing of a class of striatal neurons in Huntington’s disease. Science 230(4725):561–563
Boillee S, Vande Velde C, Cleveland DW (2006) ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 52(1):39–59. doi:10.1016/j.neuron.2006.09.018
Rando TA, Wyss-Coray T Stem cells as vehicles for youthful regeneration of aged tissues. J Gerontol A Biol Sci Med Sci 69 Suppl 1:S39-42. doi:10.1093/gerona/glu043
Orlacchio A, Bernardi G, Martino S (2010) Stem cells: an overview of the current status of therapies for central and peripheral nervous system diseases. Curr Med Chem 17(7):595–608
Sendtner M (2009) Stem cells: tailor-made diseased neurons. Nature 457(7227):269–270. doi:10.1038/457269a
Yu D, Silva GA (2008) Stem cell sources and therapeutic approaches for central nervous system and neural retinal disorders. Neurosurg Focus 24(3–4):E11. doi:10.3171/FOC/2008/24/3–4/E10
Park DH, Eve DJ (2009) Regenerative medicine: advances in new methods and technologies. Med Sci Monit 15(11):RA233–251
Galli R, Gritti A, Vescovi AL (2008) Adult neural stem cells. Methods Mol Biol 438:67–84. doi:10.1007/978–1–59745–133–8_7
Srivastava AS, Malhotra R, Sharp J, Berggren T (2008) Potentials of ES cell therapy in neurodegenerative diseases. Curr Pharm Des 14(36):3873–3879
Park IH, Lerou PH, Zhao R, Huo H, Daley GQ (2008) Generation of human-induced pluripotent stem cells. Nat Protoc 3(7):1180–1186. doi:10.1038/nprot.2008.92
Conti L, Cattaneo E, Papadimou E (2008) Novel neural stem cell systems. Expert Opin Biol Ther 8(2):153–160. doi:10.1517/14712598.8.2.153
Ma DK, Bonaguidi MA, Ming GL, Song H (2009) Adult neural stem cells in the mammalian central nervous system. Cell Res 19(6):672–682. doi:10.1038/cr.2009.56
Lindvall O, Kokaia Z, Martinez-Serrano A (2004) Stem cell therapy for human neurodegenerative disorders—how to make it work. Nat Med 10(Suppl):S42–50. doi:10.1038/nm1064
Rayment EA, Williams DJ (2010) Concise review: mind the gap: challenges in characterizing and quantifying cell- and tissue-based therapies for clinical translation. Stem Cells 28(5):996–1004. doi:10.1002/stem.416
Osanai T, Kuroda S, Sugiyama T, Kawabori M, Ito M, Shichinohe H, Kuge Y, Houkin K, Tamaki N, Iwasaki Y Therapeutic effects of intra-arterial delivery of bone marrow stromal cells in traumatic brain injury of rats—in vivo cell tracking study by near-infrared fluorescence imaging. Neurosurgery 70 (2):435–444; discussion 444. doi:10.1227/NEU.0b013e318230a795
Baker PS, Brown GC (2009) Stem-cell therapy in retinal disease. Curr Opin Ophthalmol 20(3):175–181
Enns GM, Huhn SL (2008) Central nervous system therapy for lysosomal storage disorders. Neurosurg Focus 24(3–4):E12. doi:10.3171/FOC/2008/24/3–4/E11
Martino S, di Girolamo I, Orlacchio A, Datti A (2009) MicroRNA implications across neurodevelopment and neuropathology. J Biomed Biotechnol 2009:654346. doi:10.1155/2009/654346
Naegele JR, Maisano X, Yang J, Royston S, Ribeiro E (2010) Recent advancements in stem cell and gene therapies for neurological disorders and intractable epilepsy. Neuropharmacology 58(6):855–864. doi:10.1016/j.neuropharm.2010.01.019
Lindvall O, Kokaia Z (2010) Stem cells in human neurodegenerative disorders—time for clinical translation? J Clin Invest 120(1):29–40. doi:10.1172/JCI40543
Dawson E, Mapili G, Erickson K, Taqvi S, Roy K (2008) Biomaterials for stem cell differentiation. Adv Drug Deliv Rev 60(2):215–228. doi:10.1016/j.addr.2007.08.037
Atala A (2009) Engineering organs. Curr Opin Biotechnol 20(5):575–592. doi:10.1016/j.copbio.2009.10.003
Zhong Y, Bellamkonda RV (2008) Biomaterials for the central nervous system. J R Soc Interface 5(26):957–975. doi:10.1098/rsif.2008.0071
Martino S, D’Angelo F, Armentano I, Tiribuzi R, Pennacchi M, Dottori M, Mattioli S, Caraffa A, Cerulli GG, Kenny JM, Orlacchio A (2009) Hydrogenated amorphous carbon nanopatterned film designs drive human bone marrow mesenchymal stem cell cytoskeleton architecture. Tissue Eng Part A 15(10):3139–3149. doi:10.1089/ten.TEA.2008.0552
Orive G, Anitua E, Pedraz JL, Emerich DF (2009) Biomaterials for promoting brain protection, repair and regeneration. Nat Rev Neurosci 10(9):682–692. doi:10.1038/nrn2685
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676. doi:10.1016/j.cell.2006.07.024
Takahashi J (2007) Stem cell therapy for Parkinson’s disease. Expert Rev Neurother 7(6):667–675. doi:10.1586/14737175.7.6.667
Yu SC, Yi L, Zhou ZH, Yao XH, Ping YF, Bian XW (2007) Isolation and identification of tumor stem-like cells from human glioma cell line U87 after treatment of vincristine. Ai Zheng 26(12):1388–1391
Yu Y, Gu S, Huang H, Wen T (2007) Combination of bFGF, heparin and laminin induce the generation of dopaminergic neurons from rat neural stem cells both in vitro and in vivo. J Neurol Sci 255(1–2):81–86. doi:10.1016/j.jns.2007.01.076
Hovakimyan M, Haas SJ, Schmitt O, Gerber B, Wree A, Andressen C (2008) Mesencephalic human neural progenitor cells transplanted into the adult hemiparkinsonian rat striatum lack dopaminergic differentiation but improve motor behavior. Cells Tissues Organs 188(4):373–383. doi:10.1159/000140680
Park HJ, Lee PH, Bang OY, Lee G, Ahn YH (2008) Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson’s disease. J Neurochem 107(1):141–151. doi:10.1111/j.1471–4159.2008.05589.x
Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M (2010) Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463(7284):1035–1041. doi:10.1038/nature08797
Marro S, Pang ZP, Yang N, Tsai MC, Qu K, Chang HY, Sudhof TC, Wernig M (2011) Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell 9(4):374–382. doi:10.1016/j.stem.2011.09.002
Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Sudhof TC, Wernig M (2011) Induction of human neuronal cells by defined transcription factors. Nature 476(7359):220–223. doi:10.1038/nature10202
Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR (2011) MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 476(7359):228–231. doi:10.1038/nature10323
Ebert AD, Yu J, Rose FF Jr, Mattis VB, Lorson CL, Thomson JA, Svendsen CN (2009) Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 457(7227):277–280. doi:10.1038/nature07677
Lee G, Papapetrou EP, Kim H, Chambers SM, Tomishima MJ, Fasano CA, Ganat YM, Menon J, Shimizu F, Viale A, Tabar V, Sadelain M, Studer L (2009) Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature 461(7262):402–406. doi:10.1038/nature08320
Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, Bjorklund A, Lindvall O, Jakobsson J, Parmar M (2011) Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A 108(25):10343–10348. doi:10.1073/pnas.1105135108
Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, Eggan K (2011) Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell 9(3):205–218. doi:10.1016/j.stem.2011.07.014
Marchetto MC, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, Chen G, Gage FH, Muotri AR (2010) A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 143(4):527–539. doi:10.1016/j.cell.2010.10.016
Cheung AY, Horvath LM, Grafodatskaya D, Pasceri P, Weksberg R, Hotta A, Carrel L, Ellis J (2011) Isolation of MECP2-null Rett syndrome patient hiPS cells and isogenic controls through X-chromosome inactivation. Hum Mol Genet 20(11):2103–2115. doi:10.1093/hmg/ddr093
Lujan E, Chanda S, Ahlenius H, Sudhof TC, Wernig M (2012) Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci U S A 109(7):2527–2532. doi:10.1073/pnas.1121003109
Han DW, Tapia N, Hermann A, Hemmer K, Hoing S, Arauzo-Bravo MJ, Zaehres H, Wu G, Frank S, Moritz S, Greber B, Yang JH, Lee HT, Schwamborn JC, Storch A, Scholer HR (2012) Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell 10(4):465–472. doi:10.1016/j.stem.2012.02.021
Nguyen HN, Byers B, Cord B, Shcheglovitov A, Byrne J, Gujar P, Kee K, Schule B, Dolmetsch RE, Langston W, Palmer TD, Pera RR (2011) LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell 8(3):267–280. doi:10.1016/j.stem.2011.01.013
Seibler P, Graziotto J, Jeong H, Simunovic F, Klein C, Krainc D (2011) Mitochondrial Parkin recruitment is impaired in neurons derived from mutant PINK1 induced pluripotent stem cells. J Neurosci 31(16):5970–5976. doi:10.1523/JNEUROSCI.4441–10.2011
Ambasudhan R, Talantova M, Coleman R, Yuan X, Zhu S, Lipton SA, Ding S (2011) Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell 9(2):113–118. doi:10.1016/j.stem.2011.07.002
Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V (2011) Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 476(7359):224–227. doi:10.1038/nature10284
Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, Walker D, Zhang WR, Kreitzer AC, Huang Y (2012) Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell 11(1):100–109. doi:10.1016/j.stem.2012.05.018
Hashimoto-Torii K, Torii M, Fujimoto M, Nakai A, El Fatimy R, Mezger V, Ju MJ, Ishii S, Chao SH, Brennand KJ, Gage FH, Rakic P (2014) Roles of heat shock factor 1 in neuronal response to fetal environmental risks and its relevance to brain disorders. Neuron 82(3):560–572. doi:10.1016/j.neuron.2014.03.002
Mitne-Neto M, Machado-Costa M, Marchetto MC, Bengtson MH, Joazeiro CA, Tsuda H, Bellen HJ, Silva HC, Oliveira AS, Lazar M, Muotri AR, Zatz M (2011) Downregulation of VAPB expression in motor neurons derived from induced pluripotent stem cells of ALS8 patients. Hum Mol Genet 20(18):3642–3652. doi:10.1093/hmg/ddr284
Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, McCarthy S, Sebat J, Gage FH (2011) Modelling schizophrenia using human induced pluripotent stem cells. Nature 473(7346):221–225. doi:10.1038/nature09915
Kim J, Su SC, Wang H, Cheng AW, Cassady JP, Lodato MA, Lengner CJ, Chung CY, Dawlaty MM, Tsai LH, Jaenisch R (2011) Functional integration of dopaminergic neurons directly converted from mouse fibroblasts. Cell Stem Cell 9(5):413–419. doi:10.1016/j.stem.2011.09.011
Rais Y, Zviran A, Geula S, Gafni O, Chomsky E, Viukov S, Mansour AA, Caspi I, Krupalnik V, Zerbib M, Maza I, Mor N, Baran D, Weinberger L, Jaitin DA, Lara-Astiaso D, Blecher-Gonen R, Shipony Z, Mukamel Z, Hagai T, Gilad S, Amann-Zalcenstein D, Tanay A, Amit I, Novershtern N, Hanna JH Deterministic direct reprogramming of somatic cells to pluripotency. Nature 502 (7469):65–70. doi:10.1038/nature1258
Salewski RP, Eftekharpour E, Fehlings MG (2010) Are induced pluripotent stem cells the future of cell-based regenerative therapies for spinal cord injury? J Cell Physiol 222(3):515–521. doi:10.1002/jcp.21995
Kiskinis E, Eggan K (2010) Progress toward the clinical application of patient-specific pluripotent stem cells. J Clin Invest 120(1):51–59. doi:10.1172/JCI40553
Zhang ZG, Chopp M (2009) Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol 8(5):491–500. doi:10.1016/S1474–4422(09)70061–4
Li JY, Christophersen NS, Hall V, Soulet D, Brundin P (2008) Critical issues of clinical human embryonic stem cell therapy for brain repair. Trends Neurosci 31(3):146–153. doi:10.1016/j.tins.2007.12.001
Bachoud-Levi AC, Gaura V, Brugieres P, Lefaucheur JP, Boisse MF, Maison P, Baudic S, Ribeiro MJ, Bourdet C, Remy P, Cesaro P, Hantraye P, Peschanski M (2006) Effect of fetal neural transplants in patients with Huntington’s disease 6 years after surgery: a long-term follow-up study. Lancet Neurol 5(4):303–309. doi:10.1016/S1474–4422(06)70381–7
Deda H, Inci MC, Kurekci AE, Sav A, Kayihan K, Ozgun E, Ustunsoy GE, Kocabay S (2009) Treatment of amyotrophic lateral sclerosis patients by autologous bone marrow-derived hematopoietic stem cell transplantation: a 1 year follow-up. Cytotherapy 11(1):18–25. doi:10.1080/14653240802549470
Appel SH, Engelhardt JI, Henkel JS, Siklos L, Beers DR, Yen AA, Simpson EP, Luo Y, Carrum G, Heslop HE, Brenner MK, Popat U (2008) Hematopoietic stem cell transplantation in patients with sporadic amyotrophic lateral sclerosis. Neurology 71(17):1326–1334. doi:10.1212/01.wnl.0000327668.43541.22
Akesson E, Sandelin M, Kanaykina N, Aldskogius H, Kozlova EN (2008) Long-term survival, robust neuronal differentiation, and extensive migration of human forebrain stem/progenitor cells transplanted to the adult rat dorsal root ganglion cavity. Cell Transplant 17(10–11):1115–1123
Biffi A, Lucchini G, Rovelli A, Sessa M (2008) Metachromatic leukodystrophy: an overview of current and prospective treatments. Bone Marrow Transplant 42(Suppl 2):S2–6. doi:10.1038/bmt.2008.275
Lindvall O, Kokaia Z (2006) Stem cells for the treatment of neurological disorders. Nature 441(7097):1094–1096. doi:10.1038/nature04960
Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R (2008) Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci U S A 105(15):5856–5861. doi:10.1073/pnas.0801677105
Cai J, Yang M, Poremsky E, Kidd S, Schneider JS, Iacovitti L Dopaminergic neurons derived from human induced pluripotent stem cells survive and integrate into 6-OHDA-lesioned rats. Stem Cells Dev 19 (7):1017–1023. doi:10.1089/scd.2009.0319
Hargus G, Cooper O, Deleidi M, Levy A, Lee K, Marlow E, Yow A, Soldner F, Hockemeyer D, Hallett PJ, Osborn T, Jaenisch R, Isacson O Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in parkinsonian rats. Proc Natl Acad Sci U S A 107 (36):15921–15926. doi:10.1073/pnas.1010209107
Jonsson ME, Ono Y, Bjorklund A, Thompson LH (2009) Identification of transplantable dopamine neuron precursors at different stages of midbrain neurogenesis. Exp Neurol 219(1):341–354. doi:10.1016/j.expneurol.2009.06.006
Rodriguez-Gomez JA, Lu JQ, Velasco I, Rivera S, Zoghbi SS, Liow JS, Musachio JL, Chin FT, Toyama H, Seidel J, Green MV, Thanos PK, Ichise M, Pike VW, Innis RB, McKay RD (2007) Persistent dopamine functions of neurons derived from embryonic stem cells in a rodent model of Parkinson disease. Stem Cells 25(4):918–928. doi:10.1634/stemcells.2006–0386
Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA (2006) Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med 12(11):1259–1268. doi:10.1038/nm1495
Cho MS, Lee YE, Kim JY, Chung S, Cho YH, Kim DS, Kang SM, Lee H, Kim MH, Kim JH, Leem JW, Oh SK, Choi YM, Hwang DY, Chang JW, Kim DW (2008) Highly efficient and large-scale generation of functional dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A 105(9):3392–3397. doi:10.1073/pnas.0712359105
Ben-Hur T, Idelson M, Khaner H, Pera M, Reinhartz E, Itzik A, Reubinoff BE (2004) Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in parkinsonian rats. Stem Cells 22(7):1246–1255. doi:10.1634/stemcells.2004–0094
Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sanchez-Pernaute R, Bankiewicz K, McKay R (2002) Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature 418(6893):50–56. doi:10.1038/nature00900
Nishimura F, Yoshikawa M, Kanda S, Nonaka M, Yokota H, Shiroi A, Nakase H, Hirabayashi H, Ouji Y, Birumachi J, Ishizaka S, Sakaki T (2003) Potential use of embryonic stem cells for the treatment of mouse parkinsonian models: improved behavior by transplantation of in vitro differentiated dopaminergic neurons from embryonic stem cells. Stem Cells 21(2):171–180. doi:10.1634/stemcells.21–2–171
Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, Perrier AL, Bruses J, Rubio ME, Topf N, Tabar V, Harrison NL, Beal MF, Moore MA, Studer L (2003) Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol 21(10):1200–1207. doi:10.1038/nbt870
Tabar V, Tomishima M, Panagiotakos G, Wakayama S, Menon J, Chan B, Mizutani E, Al-Shamy G, Ohta H, Wakayama T, Studer L (2008) Therapeutic cloning in individual parkinsonian mice. Nat Med 14(4):379–381. doi:10.1038/nm1732
Villaescusa JC, Arenas E (2010) Transplantable midbrain dopamine neurons: a moving target. Exp Neurol 222(2):173–178. doi:10.1016/j.expneurol.2009.12.028
Brundin P, Strecker RE, Lindvall O, Isacson O, Nilsson OG, Barbin G, Prochiantz A, Forni C, Nieoullon A, Widner H et al (1987) Intracerebral grafting of dopamine neurons. Experimental basis for clinical trials in patients with Parkinson’s disease. Ann N Y Acad Sci 495:473–496
Nishimura K, Takahashi J Therapeutic application of stem cell technology toward the treatment of Parkinson’s disease. Biol Pharm Bull 36 (2):171–175
Tarazi FI, Sahli ZT, Wolny M, Mousa SA Emerging therapies for Parkinson’s disease: from bench to bedside. Pharmacol Ther. doi:10.1016/j.pharmthera.2014.05.010
Mendez I, Sanchez-Pernaute R, Cooper O, Vinuela A, Ferrari D, Bjorklund L, Dagher A, Isacson O (2005) Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain 128(Pt 7):1498–1510. doi:10.1093/brain/awh510
Geraerts M, Krylyshkina O, Debyser Z, Baekelandt V (2007) Concise review: therapeutic strategies for Parkinson disease based on the modulation of adult neurogenesis. Stem Cells 25(2):263–270. doi:10.1634/stemcells.2006–0364
Friling S, Andersson E, Thompson LH, Jonsson ME, Hebsgaard JB, Nanou E, Alekseenko Z, Marklund U, Kjellander S, Volakakis N, Hovatta O, El Manira A, Bjorklund A, Perlmann T, Ericson J (2009) Efficient production of mesencephalic dopamine neurons by Lmx1a expression in embryonic stem cells. Proc Natl Acad Sci U S A 106(18):7613–7618. doi:10.1073/pnas.0902396106
Hallett M, Litvan I (1999) Evaluation of surgery for Parkinson’s disease: a report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. The Task Force on Surgery for Parkinson’s Disease. Neurology 53(9):1910–1921
Hallett M, Litvan I (2000) Scientific position paper of the Movement Disorder Society evaluation of surgery for Parkinson’s disease. Task Force on Surgery for Parkinson’s Disease of the American Academy of Neurology Therapeutic and Technology Assessment Committee. Mov Disord 15(3):436–438
Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S (2001) Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med 344(10):710–719. doi:10.1056/NEJM200103083441002
Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB (2003) A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol 54(3):403–414. doi:10.1002/ana.10720
Bjorklund A, Dunnett SB, Brundin P, Stoessl AJ, Freed CR, Breeze RE, Levivier M, Peschanski M, Studer L, Barker R (2003) Neural transplantation for the treatment of Parkinson’s disease. Lancet Neurol 2(7):437–445
Freed CR, Leehey MA, Zawada M, Bjugstad K, Thompson L, Breeze RE (2003) Do patients with Parkinson’s disease benefit from embryonic dopamine cell transplantation? J Neurol 250(Suppl 3):III44–46. doi:10.1007/s00415–003–1308–5
Freed CR, Breeze RE, Fahn S, Eidelberg D (2004) Preoperative response to levodopa is the best predictor of transplant outcome. Ann Neurol 55 (6):896; author reply 896–897. doi:10.1002/ana.20085
Byers B, Cord B, Nguyen HN, Schule B, Fenno L, Lee PC, Deisseroth K, Langston JW, Pera RR, Palmer TD (2011) SNCA triplication Parkinson’s patient’s iPSC-derived DA neurons accumulate alpha-synuclein and are susceptible to oxidative stress. PLoS One 6(11):e26159. doi:10.1371/journal.pone.0026159
Sagal J, Zhan X, Xu J, Tilghman J, Karuppagounder SS, Chen L, Dawson VL, Dawson TM, Laterra J, Ying M Proneural transcription factor Atoh1 drives highly efficient differentiation of human pluripotent stem cells into dopaminergic neurons. Stem Cells Transl Med. doi:10.5966/sctm.2013–0213
Thies W, Bleiler L, Alzheimer’s A (2013) 2013 Alzheimer’s disease facts and figures. Alzheimers Dement 9(2):208–245. doi:10.1016/j.jalz.2013.02.003
Gurav AN (2014) Alzheimer’s disease and periodontitis—an elusive link. Rev Assoc Med Bras 60(2):173–180
Hansen N (2014) Brain stimulation for combating Alzheimer’s disease. Front Neurol 5:80. doi:10.3389/fneur.2014.00080
Babaei P, Soltani Tehrani B, Alizadeh A Transplanted bone marrow mesenchymal stem cells improve memory in rat models of Alzheimer’s disease. Stem Cells Int 2012:369417. doi:10.1155/2012/369417
Lee JK, Jin HK, Bae JS (2009) Bone marrow-derived mesenchymal stem cells reduce brain amyloid-beta deposition and accelerate the activation of microglia in an acutely induced Alzheimer’s disease mouse model. Neurosci Lett 450(2):136–141. doi:10.1016/j.neulet.2008.11.059
Park D, Lee HJ, Joo SS, Bae DK, Yang G, Yang YH, Lim I, Matsuo A, Tooyama I, Kim YB, Kim SU Human neural stem cells over-expressing choline acetyltransferase restore cognition in rat model of cognitive dysfunction. Exp Neurol 234 (2):521–526. doi:10.1016/j.expneurol.2011.12.040
Park DH, Eve DJ, Sanberg PR, Musso J, 3rd, Bachstetter AD, Wolfson A, Schlunk A, Baradez MO, Sinden JD, Gemma C Increased neuronal proliferation in the dentate gyrus of aged rats following neural stem cell implantation. Stem Cells Dev 19 (2):175–180. doi:10.1089/scd.2009.0172
Qu T, Brannen CL, Kim HM, Sugaya K (2001) Human neural stem cells improve cognitive function of aged brain. Neuroreport 12(6):1127–1132
Kern DS, Maclean KN, Jiang H, Synder EY, Sladek JR, Jr., Bjugstad KB Neural stem cells reduce hippocampal tau and reelin accumulation in aged Ts65Dn Down syndrome mice. Cell Transplant 20 (3):371–379. doi:10.3727/096368910X528085
Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Muller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM (2009) Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A 106(32):13594–13599. doi:10.1073/pnas.0901402106
Yamasaki TR, Blurton-Jones M, Morrissette DA, Kitazawa M, Oddo S, LaFerla FM (2007) Neural stem cells improve memory in an inducible mouse model of neuronal loss. J Neurosci 27(44):11925–11933. doi:10.1523/JNEUROSCI.1627–07.2007
Xuan AG, Luo M, Ji WD, Long DH (2009) Effects of engrafted neural stem cells in Alzheimer’s disease rats. Neurosci Lett 450(2):167–171. doi:10.1016/j.neulet.2008.12.001
Kim S, Chang KA, Kim J, Park HG, Ra JC, Kim HS, Suh YH The preventive and therapeutic effects of intravenous human adipose-derived stem cells in Alzheimer’s disease mice. PLoS One 7 (9):e45757. doi:10.1371/journal.pone.0045757
Wang Q, Matsumoto Y, Shindo T, Miyake K, Shindo A, Kawanishi M, Kawai N, Tamiya T, Nagao S (2006) Neural stem cells transplantation in cortex in a mouse model of Alzheimer’s disease. J Med Invest 53(1–2):61–69
Esmaeilzade B, Nobakht M, Joghataei MT, Rahbar Roshandel N, Rasouli H, Samadi Kuchaksaraei A, Hosseini SM, Najafzade N, Asalgoo S, Hejazian LB, Moghani Ghoroghi F Delivery of epidermal neural crest stem cells (EPI-NCSC) to hippocamp in Alzheimer’s disease rat model. Iran Biomed J 16 (1):1–9
Lee GJ, Lu PH, Medina LD, Rodriguez-Agudelo Y, Melchor S, Coppola G, Braskie MN, Hua X, Apostolova LG, Leow AD, Thompson PM, Ringman JM Regional brain volume differences in symptomatic and presymptomatic carriers of familial Alzheimer’s disease mutations. J Neurol Neurosurg Psychiatry 84 (2):154–162. doi:10.1136/jnnp-2011–302087
Wu L, Sluiter AA, Guo HF, Balesar RA, Swaab DF, Zhou JN, Verwer RW (2008) Neural stem cells improve neuronal survival in cultured postmortem brain tissue from aged and Alzheimer patients. J Cell Mol Med 12(5A):1611–1621. doi:10.1111/j.1582–4934.2007.00203.x
Sugaya K, Merchant S (2008) How to approach Alzheimer’s disease therapy using stem cell technologies. J Alzheimers Dis 15(2):241–254
Waldau B, Shetty AK (2008) Behavior of neural stem cells in the Alzheimer brain. Cell Mol Life Sci 65(15):2372–2384. doi:10.1007/s00018–008–8053-y
Ying QL, Stavridis M, Griffiths D, Li M, Smith A (2003) Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol 21(2):183–186. doi:10.1038/nbt780
Xu Y, Tamamaki N, Noda T, Kimura K, Itokazu Y, Matsumoto N, Dezawa M, Ide C (2005) Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol 192(2):251–264. doi:10.1016/j.expneurol.2004.12.021
Moghadam FH, Alaie H, Karbalaie K, Tanhaei S, Nasr Esfahani MH, Baharvand H (2009) Transplantation of primed or unprimed mouse embryonic stem cell-derived neural precursor cells improves cognitive function in Alzheimerian rats. Differentiation 78(2–3):59–68. doi:10.1016/j.diff.2009.06.005
Tang J, Xu H, Fan X, Li D, Rancourt D, Zhou G, Li Z, Yang L (2008) Embryonic stem cell-derived neural precursor cells improve memory dysfunction in Abeta (1–40) injured rats. Neurosci Res 62(2):86–96. doi:10.1016/j.neures.2008.06.005
Schwartz CM, Tavakoli T, Jamias C, Park SS, Maudsley S, Martin B, Phillips TM, Yao PJ, Itoh K, Ma W, Rao MS, Arenas E, Mattson MP Stromal factors SDF1alpha, sFRP1, and VEGFD induce dopaminergic neuron differentiation of human pluripotent stem cells. J Neurosci Res 90 (7):1367–1381. doi:10.1002/jnr.23064
Cho EG, Zaremba JD, McKercher SR, Talantova M, Tu S, Masliah E, Chan SF, Nakanishi N, Terskikh A, Lipton SA MEF2C enhances dopaminergic neuron differentiation of human embryonic stem cells in a parkinsonian rat model. PLoS One 6 (8):e24027. doi:10.1371/journal.pone.0024027PONE-D-11–07261
Liu Y, Weick JP, Liu H, Krencik R, Zhang X, Ma L, Zhou GM, Ayala M, Zhang SC Medial ganglionic eminence-like cells derived from human embryonic stem cells correct learning and memory deficits. Nat Biotechnol 31 (5):440–447. doi:10.1038/nbt.2565
Fan X, Sun D, Tang X, Cai Y, Yin ZQ, Xu H Stem-cell challenges in the treatment of Alzheimer’s disease: a long way from bench to bedside. Med Res Rev. doi:10.1002/med.21309
Mohamet L, Miazga NJ, Ward CM Familial Alzheimer’s disease modelling using induced pluripotent stem cell technology. World J Stem Cells 6 (2):239–247. doi:10.4252/wjsc.v6.i2.239
Yagi T, Ito D, Okada Y, Akamatsu W, Nihei Y, Okano H, Suzuki N [Modeling familial Alzheimer’s disease with induced pluripotent stem cells]. Rinsho Shinkeigaku 52 (11):1134–1136
Yagi T, Ito D, Okada Y, Akamatsu W, Nihei Y, Yoshizaki T, Yamanaka S, Okano H, Suzuki N Modeling familial Alzheimer’s disease with induced pluripotent stem cells. Hum Mol Genet 20 (23):4530–4539. doi:10.1093/hmg/ddr394
Yahata N, Asai M, Kitaoka S, Takahashi K, Asaka I, Hioki H, Kaneko T, Maruyama K, Saido TC, Nakahata T, Asada T, Yamanaka S, Iwata N, Inoue H Anti-Abeta drug screening platform using human iPS cell-derived neurons for the treatment of Alzheimer’s disease. PLoS One 6 (9):e25788. doi:10.1371/journal.pone.0025788PONE-D-11–09488
Kremer B, Goldberg P, Andrew SE, Theilmann J, Telenius H, Zeisler J, Squitieri F, Lin B, Bassett A, Almqvist E et al (1994) A worldwide study of the Huntington’s disease mutation. The sensitivity and specificity of measuring CAG repeats. N Engl J Med 330(20):1401–1406. doi:10.1056/NEJM199405193302001
Walker FO (2007) Huntington’s disease. Lancet 369(9557):218–228. doi:10.1016/S0140–6736(07)60111–1
Im W, Kim M (2014) Cell Therapy strategies vs. paracrine effect in Huntington’s disease. J Mov Disord 7 (1):1–6. doi:10.14802/jmd.14001
Visnyei K, Tatsukawa KJ, Erickson RI, Simonian S, Oknaian N, Carmichael ST, Kornblum HI (2006) Neural progenitor implantation restores metabolic deficits in the brain following striatal quinolinic acid lesion. Exp Neurol 197(2):465–474. doi:10.1016/j.expneurol.2005.10.023
Vazey EM, Chen K, Hughes SM, Connor B (2006) Transplanted adult neural progenitor cells survive, differentiate and reduce motor function impairment in a rodent model of Huntington’s disease. Exp Neurol 199(2):384–396. doi:10.1016/j.expneurol.2006.01.034
Amin EM, Reza BA, Morteza BR, Maryam MM, Ali M, Zeinab N (2008) Microanatomical evidences for potential of mesenchymal stem cells in amelioration of striatal degeneration. Neurol Res 30(10):1086–1090. doi:10.1179/174313208X327955
Juopperi TA, Kim WR, Chiang CH, Yu H, Margolis RL, Ross CA, Ming GL, Song H Astrocytes generated from patient induced pluripotent stem cells recapitulate features of Huntington’s disease patient cells. Mol Brain 5:17. doi:10.1186/1756–6606–5–17
Fink KD, Crane AT, Leveque X, Dues DJ, Huffman LD, Moore AC, Story DT, Dejonge RE, Antcliff A, Starski PA, Lu M, Lescaudron L, Rossignol J, Dunbar GL Intrastriatal transplantation of adenovirus-generated induced pluripotent stem cells for treating neuropathological and functional deficits in a rodent model of Huntington’s disease. Stem Cells Transl Med 3 (5):620–631. doi:10.5966/sctm.2013–0151
Traxinger K, Kelly C, Johnson BA, Lyles RH, Glass JD (2013) Prognosis and epidemiology of amyotrophic lateral sclerosis: analysis of a clinic population, 1997–2011. Neurol Clin Pract 3(4):313–320. doi:10.1212/CPJ.0b013e3182a1b8ab
Vucic S, Rothstein JD, Kiernan MC (2014) Advances in treating amyotrophic lateral sclerosis: insights from pathophysiological studies. Trends Neurosci. doi:10.1016/j.tins.2014.05.006
Burrell JR, Vucic S, Kiernan MC (2011) Isolated bulbar phenotype of amyotrophic lateral sclerosis. Amyotroph Lateral Scler 12(4):283–289. doi:10.3109/17482968.2011.551940
Wolfson C, Kilborn S, Oskoui M, Genge A (2009) Incidence and prevalence of amyotrophic lateral sclerosis in Canada: a systematic review of the literature. Neuroepidemiology 33(2):79–88. doi:10.1159/000222089
Sun H, Hou Z, Yang H, Meng M, Li P, Zou Q, Yang L, Chen Y, Chai H, Zhong H, Yang ZZ, Zhao J, Lai L, Jiang X, Xiao Z Multiple systemic transplantations of human amniotic mesenchymal stem cells exert therapeutic effects in an ALS mouse model. Cell Tissue Res. doi:10.1007/s00441–014–1903-z
Boido M, Piras A, Valsecchi V, Spigolon G, Mareschi K, Ferrero I, Vizzini A, Temi S, Mazzini L, Fagioli F, Vercelli A. Human mesenchymal stromal cell transplantation modulates neuroinflammatory milieu in a mouse model of amyotrophic lateral sclerosis. Cytotherapy. doi:10.1016/j.jcyt.2014.02.003
Chestkov IV, Vasilieva EA, Illarioshkin SN, Lagarkova MA, Kiselev SL Patient-specific induced pluripotent stem cells for SOD1-associated amyotrophic lateral sclerosis pathogenesis studies. Acta Naturae 6 (1):54–60
Nizzardo M, Simone C, Rizzo F, Ruggieri M, Salani S, Riboldi G, Faravelli I, Zanetta C, Bresolin N, Comi GP, Corti S Minimally invasive transplantation of iPSC-derived ALDHhiSSCloVLA4+ neural stem cells effectively improves the phenotype of an amyotrophic lateral sclerosis model. Hum Mol Genet 23 (2):342–354. doi:10.1093/hmg/ddt425
Pan T, Kondo S, Le W, Jankovic J (2008) The role of autophagy–lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain 131(Pt 8):1969–1978. doi:10.1093/brain/awm318
Lattanzi A, Neri M, Maderna C, di Girolamo I, Martino S, Orlacchio A, Amendola M, Naldini L, Gritti A (2010) Widespread enzymatic correction of CNS tissues by a single intracerebral injection of therapeutic lentiviral vector in leukodystrophy mouse models. Hum Mol Genet 19(11):2208–2227. doi:10.1093/hmg/ddq099
Martino S, di Girolamo I, Cavazzin C, Tiribuzi R, Galli R, Rivaroli A, Valsecchi M, Sandhoff K, Sonnino S, Vescovi A, Gritti A, Orlacchio A (2009) Neural precursor cell cultures from GM2 gangliosidosis animal models recapitulate the biochemical and molecular hallmarks of the brain pathology. J Neurochem 109(1):135–147. doi:10.1111/j.1471–4159.2009.05919.x
Martino S, Marconi P, Tancini B, Dolcetta D, De Angelis MG, Montanucci P, Bregola G, Sandhoff K, Bordignon C, Emiliani C, Manservigi R, Orlacchio A (2005) A direct gene transfer strategy via brain internal capsule reverses the biochemical defect in Tay-Sachs disease. Hum Mol Genet 14(15):2113–2123. doi:10.1093/hmg/ddi216
Biffi A, Cesani M, Fumagalli F, Del Carro U, Baldoli C, Canale S, Gerevini S, Amadio S, Falautano M, Rovelli A, Comi G, Roncarolo MG, Sessa M (2008) Metachromatic leukodystrophy—mutation analysis provides further evidence of genotype-phenotype correlation. Clin Genet 74(4):349–357. doi:10.1111/j.1399–0004.2008.01058.x
Hu YF, Gourab K, Wells C, Clewes O, Schmit BD, Sieber-Blum M (2010) Epidermal neural crest stem cell (EPI-NCSC)-mediated recovery of sensory function in a mouse model of spinal cord injury. Stem Cell Rev 6(2):186–198. doi:10.1007/s12015–010–9152–3
Siatskas C, Bernard CC (2009) Stem cell and gene therapeutic strategies for the treatment of multiple sclerosis. Curr Mol Med 9(8):992–1016
Bithell A, Williams BP (2005) Neural stem cells and cell replacement therapy: making the right cells. Clin Sci (Lond) 108(1):13–22. doi:10.1042/CS20040276->
Hermann A, Gastl R, Liebau S, Popa MO, Fiedler J, Boehm BO, Maisel M, Lerche H, Schwarz J, Brenner R, Storch A (2004) Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J Cell Sci 117(Pt 19):4411–4422. doi:10.1242/jcs.01307
Jang YK, Park JJ, Lee MC, Yoon BH, Yang YS, Yang SE, Kim SU (2004) Retinoic acid-mediated induction of neurons and glial cells from human umbilical cord-derived hematopoietic stem cells. J Neurosci Res 75(4):573–584. doi:10.1002/jnr.10789
Joannides A, Gaughwin P, Schwiening C, Majed H, Sterling J, Compston A, Chandran S (2004) Efficient generation of neural precursors from adult human skin: astrocytes promote neurogenesis from skin-derived stem cells. Lancet 364(9429):172–178. doi:10.1016/S0140–6736(04)16630–0
McGuckin CP, Forraz N, Allouard Q, Pettengell R (2004) Umbilical cord blood stem cells can expand hematopoietic and neuroglial progenitors in vitro. Exp Cell Res 295(2):350–359. doi:10.1016/j.yexcr.2003.12.028
Acknowledgments
We thank our lab members for critical reading of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors’ Contributions
GU wrote the manuscript; SS and RKS edited the manuscript. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Upadhyay, G., Shankar, S. & Srivastava, R.K. Stem Cells in Neurological Disorders: Emerging Therapy with Stunning Hopes. Mol Neurobiol 52, 610–625 (2015). https://doi.org/10.1007/s12035-014-8883-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8883-6