Abstract
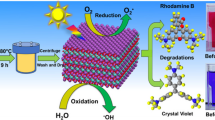
Solid-state reaction method was opted for the preparation of bismuth tungstates (Bi2WO6) in the stoichiometric ratio. The structural characterization related that the material has got orthorhombic symmetry. The high-energy ball milling did not show any structural change, but a reduction in grain size was observed from 100 to 34 nm after 5 h. The higher activity for the decolourization of rhodamine B (RHB) and methylene blue (MB) in the presence of UV light has been studied by employing Bi2WO6 as a catalyst. The dye degradation was observed by a decrease in the absorption spectrum and decolourization in the presence of UV irradiation. The degradation efficiency was found to be dependent on the size of the catalyst added in the dye solution, which may be due to increased surface area that increased the number of active sites for the reaction. The degradation efficiency of the unmilled and 5-h ball milled (Bi2WO6) catalyst was observed to be 32 and 90% in RHB, respectively. While in MB, 24 and 49% degradation efficiency was achieved by unmilled and 5-h ball milled (Bi2WO6) catalyst. The degradation rate coefficient was found to be in the decreasing order of RHB > MB, which pursued the first-order kinetic mechanism. Therefore, Bi2WO6 can act as a catalyst for the treatment of noxious and imperishable organic pollutants in water.






Similar content being viewed by others
References
Nestmann E R, Douglas G R, Matula T I, Grant C E and Kowbel D J 1979 Cancer Res. 39 4412
Chandler J E, Harrison C M and Canal A M 2000 Theriogenology 54 261
Youssef N A, Shaban S A, Ibrahim F A and Mahmoud A S 2016 Egypt. J. Pet. 25 317
Rabah M A 2008 J. Waste Manag. 28 318
Coolidge W D 1913 Phys. Rev. 2 409
Van Uitert L G and Preziosi S 1962 Int. J. Appl. Phys. 33 2908
Lecoq P, Dafinei I, Auffray E, Schneegans M, Korzhik M V, Missevitch O V et al 1995 Nucl. Instrum. Methods Phys. Res. A 365 291
Baccaro S, Borgia B, Cecilia A, Dafinei I, Diemoz M, Nikl M et al 1998 Radiat. Phys. Chem. 52 635
Treadaway M J and Powell R C 1975 Phys. Rev. 11 862
Chen W, Inagawa Y, Omatsu T, Tateda M, Takeuchi N and Usuki Y 2001 Opt. Commun. 194 401
Ehrenberg H, Weitzel H, Heid C, Fuess H, Wltschek G, Kroener T et al 1997 J. Condens. Matter Phys. 9 3189
Nagirnyi V, Feldbach E, Jönsson L, Kirm M, Lushchik A, Lushchik C et al 1998 Radiat. Meas. 29 247
Raj A E S, Mallika C, Sreedharan O M and Nagaraja K S 2002 Mater. Lett. 53 316
Garcia-Perez U M, Martinez-de La Cruz A and Peral J 2012 Electrochim. Acta 81 227
Sha Z, Sun J, Chan H S O, Jaenicke S and Wu J 2014 RSC Adv. 4 64977
Rahimi-Nasrabadi M, Pourmortazavi S M, Aghazadeh M, Ganjali M R, Karimi M S and Novrouzi P 2017 J. Mater. Sci.: Mater. Electron. 28 3780
López X A, Fuentes A F, Zaragoza M M, Guillén J A D, Gutiérrez J S, Ortiz A L et al 2016 Int. J. Hydrog. Energy. 41 23312
Amano F, Nogami K, Abe R and Ohtani B 2008 J. Phys. Chem. 112 9320
Amano F, Nogami K and Ohtani B 2009 J. Phys. Chem. 113 1536
Dai X J, Luo Y S, Zhang W D and Fu S Y 2010 Dalton Trans. 39 3426
Hu T, Li H, Zhang R, Du N and Hou W 2016 RSC Adv. 6 31744
Issarapanacheewin S, Wetchakun K, Phanichphant S, Kangwansupamonkon W and Wetchakun N 2016 Ceram. Int. 42 16007
Zhu S, Xu T, Fu H, Zhao J and Zhu Y 2007 Environ. Sci. Technol. 41 6234
Zhang L, Wang H, Chen Z, Wong P K and Liu J 2011 Appl. Catal. B 106 1
Yu J, Xiong J, Cheng B, Yu Y and Wang J 2005 J. Solid State Chem. 178 1968
Xia J, Li H, Luo Z, Xu H, Wang K, Yin S et al 2010 Mater. Chem. Phys. 121 6
Zhao G, Liu S, Lu Q and Song L 2012 Ind. Eng. Chem. 51 10307
Zhu Y, Wang Y, Ling Q and Zhu Y 2017 Appl. Catal. B 200 222
Xiao J, Dong W, Song C, Yu Y, Zhang L, Li C et al 2015 Mater. Sci. Semicond. Process. 40 463
Phuruangrat A, Dumrongrojthanath P, Ekthammathat N, Thongtem S and Thongtem T 2014 J. Nanomater. 2014 Article ID 138561
Nobre F X, Junior W A G P, Ruiz Y L, Bentes V L I, Silva-Moraes M O, Silva T M C et al 2019 Mater. Res. Bull. 109 60
Maczka M, Hanuza J, Paraguassu W, Gomes Souza Filho A, Tarso Cavalcante Freire P and Mendes Filho J 2008 Appl. Phys. Lett. 92 112911
Fu H, Pan C, Zhang L and Zhu Y 2007 Mater. Res. Bull. 42 696
Frost R L, Duong L and Weier M 2004 Spectrochim. Acta A 60 1853
Kania A, Niewiadomski A and Kugel G E 2013 Phase Transit. 86 290
Mishra R K, Weibel M, Müller T, Heinz H and Flatt R J 2017 Chimia 71 451
Maczka M, Paraguassu W, Souza Filho A G, Freire P T C, Mendes Filho J and Hanuza J 2008 Phys. Rev. 77 094137
Tkalčević M 2016 Doctoral dissertation (University of Zagreb, Faculty of Chemical Engineering and Technology)
Zhou Y, Zhang Y, Lin M, Long J, Zhang Z, Lin H et al 2015 Nat. Commun. 6 1
Loyalka S K and Riggs C A 1995 J. Appl. Spectrosc. 49 1107
Fujihara K, Izumi S, Ohno T and Matsumura M 2000 J. Photochem. Photobiol. A 132 99
Ma Y and Yao J N 1998 J. Photochem. Photobiol. A Chem. 116 167
Watanabe T, Takizawa T and Honda K 1977 J. Phys. Chem. Lett. 81 1845
Takizawa T, Watanabe T and Honda K 1978 J. Phys. Chem. Lett. 82 1391
López S M, Hidalgo M C, Navío J A and Colón G 2011 J. Hazard. Mater. 185 1425
Xian T, Yang H, Xian W, Chen X F and Dai J F 2013 Prog. React. Kinet. Mec. 38 417
Liu W, Wang M, Xu C and Chen S 2012 Chem. Eng. J. 209 386
Wang B, Yang H, Xian T, Di L J, Li R S and Wang X X 2015 J. Nanomater Article ID 146327
Tanaka K, Padermpole K and Hisanaga T 2000 Water Res. 34 327
Gouvea C A, Wypych F, Moraes S G, Duran N, Nagata N and Peralta-Zamora P 2000 Chemosphere 40 433
Konstantinou I K and Albanis T A 2004 Appl. Catal. B 49 1
Fu H, Zhang L, Yao W and Zhu Y 2006 Appl. Catal. B: Environ. 66 100
Morrison S R 1980 Electrochemical at semiconductor and oxidized metal electrodes (New York: Plenum)
Andersen T, Haugen H K and Hotop H 1999 J. Phys. Chem. Ref. Data 28 1511
Dung D, Ramsden J and Gratzel M 1982 J. Am. Chem. Soc. 104 2977
Tachikawa T, Fujitsuka M and Majima T 2007 J. Phys. Chem. 111 5259
Weast R C 1988 Handbook of chemistry and physics 1st edn (Boca Raton, Florida: CRC Press) p 69
Acknowledgements
One of the author (SK) thanks the Department of Science and Technology, Government of India, for providing financial assistance through the WOS-A Fellowship (SR/WOS-A/CS-128/2018) to carry out the research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khanam, S., Rout, S.K. Decolourization of rhodamine B and methylene blue dyes in the presence of bismuth tungstates: a detailed investigation on the effect of grain size. Bull Mater Sci 44, 2 (2021). https://doi.org/10.1007/s12034-020-02292-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-020-02292-3