Abstract
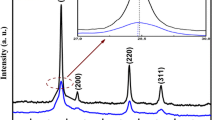
Currently, copper chromium oxide crystallizing in delafossite structure attracts huge research interest due to its versatile applications arising from its layered structure. In this work, delafossite CuCrO2 was synthesized by sol–gel method from their respective hydrated nitrate salts with citric acid as a chelating agent. The phase formation temperature was found to be between 750 and 775∘C. At 750∘C, the partial formation of delafossite CuCrO2 spheres with particle size in nano-regime was observed in the midst of platelets of spinel CuCr2O4. A green coloured powder with particle size 125–350 nm exhibiting distorted spheres was obtained at 775∘C. The increase in temperature has a profound impact on the particle size, morphology and the optical properties of CuCrO2. The X-ray powder diffraction studies revealed the formation of 3R-CuCrO2 phase (rhombohedral, space group R-3m) as a major product in the temperature range 775–1000∘C. The unit cell parameters were found to be a = b = 2.9711 Å and c = 17.0723 Å at 1000∘C. Scanning electron micrographs illustrated the different morphologies from spheres to hexagonal form via distorted spheres and cubes. The UV–Vis diffuse reflectance spectra measured for the powders exhibited semiconductor characteristics with an interesting size-related and temperature-dependent bandgap.




Similar content being viewed by others
References
Marquardt M A, Ashmore N A and Cann D P 2006 Thin Solid Films 496 146
Amrute A P, Larrazabal G O, Mondelli C and Ramirez J P 2013 Angew. Chem. Int. Ed. 52 9772
Zhou S, Fang X, Deng Z, Li D, Dong W, Tao R, Meng G and Wang T 2009 Sensor Actuat. B 143 119
Kameoka S, Okada M and Tsai A P 2008 Catal. Lett. 120 252
Meng Q, Lu S, Lu S and Xiang Y 2012 J. Sol–Gel Sci. Technol. 63 1
Powar S, Xiong D, Daeneke T, Ma M T, Gupta A, Lee G, Makuta S, Tachibana Y, Chen W, Spiccia L, Cheng Y B, Goetz G, Baeuerle P and Bach U 2014 J. Phys. Chem. C 118 16375
Xiong D, Zhang W, Zeng X, Xu Z, Chen W, Cui J, Wang M, Sun L and Cheng Y B 2013 ChemSusChem 6 1432
Shannon R D, Rogers D B and Prewitt C T 1971 Inorg. Chem. 10 713
Ketir W, Saadi S and Trari M 2012 J. Solid State Eletrochem. 16 213
Amrute A P, Lodziana Z, Mondelli C, Krumeich F and Ramirez J P 2014 Chem. Mater. 25 4423
Sheets W C, Mugnier E, Barnabe A, Marks T J and Poeppelmeier K R 2006 Chem. Mater. 18 7
Zhou S, Fang X, Deng Z, Li D, Dong W, Tao R, Meng G, Wang T and Zhu X 2008 J. Cryst. Growth 310 5375
Miclau M, Ursu D, Kumar S and Grozescu I 2012 J. Nanopart. Res. 14 1110
Chiu T W, Yu B S, Wang Y R, Chen K T and Lin Y T 2011 J. Alloys Compd. 509 2933
Deng Z, Zhu X, Tao R, Dong W and Fang X 2007 Mater. Lett. 61 686
Kumar S, Marinela S, Miclau M and Martin C 2012 Mater. Lett. 70 40
Okuda T, Jufuku N, Hidaka S and Terada N 2005 Phy. Rev. B 72 144403(1)
Poienar M, Hardy V, Kundys B, Singh K, Maignan A, Damay F and Martin C 2012 J. Solid State Chem. 185 56
Goetzendoerfer S, Polenzky C, Ulrich S and Loebmann P 2009 Thin Solid Films 518 1153
Kawazoe H, Yasukawa M, Hyodo H, Kurita M, Yanagi H and Hosono H 1997 Nature 389 939
Nagarajan R, Draeske A D, Sleight A W and Tate J 2001 J. Appl. Phys. 89 8022
Benko F A and Koffyberg F P 1986 Mater. Res. Bull. 21 753
Mahapatra S and Shivasankar S A 2003 Chem. Vap. Deposition 9 238
Li D, Fang X D, Deng Z H, Zhou S, Tao R H, Dong W W, Wang T, Zhao Y P, Meng G and Zhu X B 2007 J. Phys. D : Appl. Phys. 40 4910
Scanlon D O and Watson G W 2011 J. Mater. Chem. 21 3655
Bywalez R, Goetzendoerfer S and Loebmann P 2010 J. Mater. Chem. 20 6562
Li D, Fang X, Zhao A, Deng Z, Dong W and Tao R 2010 Vacuum 84 851
Xiong D, Zeng X, Zhang W, Chen W, Xu X, Wang M and Cheng Y B 2012 J. Mater. Chem. 22 24760
Ursu D and Miclau M 2014 J. Nanopart. Res. 16 2160
Srinivasan R, Chavillon B, Dossier-Brochard C, Cario L, Paris M, Gautron E, Deniard P, Odobel F and Jobic S 2008 J. Mater. Chem. 18 5647
Chavillon B, Cario L, Dossier-Brochard C, Srinivasan R, Le Pleux L, Pellegrin Y, Blart E, Odobel F and Jobic S 2010 Phys. Status Solidi A 207 1642
Goetzendoerfer S, Bywalez R and Loebmann P 2009 J. Sol–Gel Sci. Technol. 52 113
Ahmad A, Jagadale T, Dhas V, Khan S, Patil S, Pasricha R, Ravi V and Ogale S B 2007 Adv. Mater. 19 3295
Srinivasan R and Bolloju S 2014 AIP Conf. Proc. 1576 205
Singh K A and Pathak L C 2007 Ceram. Int. 33 1463
Saadi S, Bouguelia A and Trari M 2006 Sol. Energy 80 272
Zeghbroeck B V 2011 Principle of semiconductor devices, http://ecee. colorado.edu/~bart/book/book/contents.htm (University of Colorado)
Acknowledgements
Department of Science and Technology (DST SERB) is acknowledged for funding through the FASTTRACK scheme (No. SR/FT/CS-024/2010). We wish to thank the Department of Physics, Alagappa University at Karaikudi, Centre for Nano Science and Technology, Karunya University at Coimbatore and University of Hyderabad for characterization facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
BOLLOJU, S., SRINIVASAN, R. Sub-micron-sized delafossite CuCrO2 with different morphologies synthesized by nitrate–citric acid sol–gel route. Bull Mater Sci 40, 195–199 (2017). https://doi.org/10.1007/s12034-016-1340-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-016-1340-6