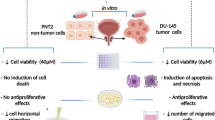
Abstract
Prostate cancer incidences are rising worldwide at an alarming rate. Drug resistance and relapse are two major challenges in the treatment of prostate cancer. Therefore, new multimodal, safe, and effective therapeutic agents are urgently required which could effectively mitigate the menace of tumor recurrence and chemo-resistance. Plant-derived products are increasingly being utilized due to their antioxidant, antibacterial, and anti-tumor potential. In the current study, 3-acetyl-11-keto-β-boswellic acid, a triterpenoid isolated from plant Boswellia, was utilized to ascertain its chemotherapeutic potential against human prostate cancer cells. Various in vitro assays including cell viability, nuclear staining, mitochondria potential, reactive oxygen species (ROS) generation, and quantification of apoptosis, were performed for the evaluation of the cytotoxic potential of AKBA. We observed that AKBA (10–50 µM) dose-dependently suppressed cell proliferation and caused programmed cell death in PC3 cells via both intrinsic and extrinsic pathway. Intriguingly, AKBA was also found to chemosensitize PC3 cells in synergistic combination with doxorubicin. To the best of our knowledge, this is the first study to document the synergistic chemosensitizing impact of AKBA when combined with doxorubicin in prostate cancer cells.This showcases the potential of AKBA in combinatorial therapy or adjuvant therapy for the management of prostate cancer. In sum, our results suggested that AKBA is a promising drug-like molecule against prostate cancer. Our investigation introduces a novel perspective, elucidating a previously unexplored dimension, and uncovering a compelling chemosensitizing phenomenon along with a strong synergistic effect arising from the concurrent application of these two agents.







Similar content being viewed by others
Data Availability
All datasets generated for this study are included in the article. Further inquiries can be directed to the corresponding author.
References
Gandaglia, G., Leni, R., Bray, F., Fleshner, N., Freedland, S. J., Kibel, A., Stattin, P., Van Poppel, H., & La Vecchia, C. (2021). Epidemiology and prevention of prostate cancer. European Urology Oncology, 4(6), 877–892.
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249.
Marhold, M., Kramer, G., Krainer, M., & Le Magnen, C. (2022). The prostate cancer landscape in Europe: Current challenges, future opportunities. Cancer Letters, 526, 304–310.
Siegel, R. L., Miller, K. D., Wagle, N. S., & Jemal, A. (2023). Cancer statistics, 2023. CA: A Cancer Journal for Clinicians, 73(1), 17–48.
Mathur, P., Sathishkumar, K., Chaturvedi, M., Das, P., Sudarshan, K. L., Santhappan, S., Nallasamy, V., John, A., Narasimhan, S., & Roselind, F. S. (2020). Cancer statistics, 2020: Report from national cancer registry programme. India. JCO Global Oncology., 6, 1063–1075.
Sathishkumar, K., Chaturvedi, M., Das, P., Stephen, S., & Mathur, P. (2022). Cancer incidence estimates for 2022 & projection for 2025: Result from national cancer Registry Programme, India. Indian Journal of Medical Research, 156(4–5), 598.
Hariharan, K., & Padmanabha, V. (2016). Demography and disease characteristics of prostate cancer in India. Indian Journal of Urology: Journal of the Urological Society of India, 32(2), 103.
Alnuqaydan, A. M., Almutary, A. G., Alshehri, O. Y., Henidi, H. A., Alajlan, A. M., Al Tamim, A., Alowaifeer, A., Rather, M. Y., & Rah, B. (2022). Evaluation of the cytotoxic activity of Tamarix articulata and its anticancer potential in prostate cancer cells. Journal of Applied Pharmaceutical Science., 12(02), 89–108.
Amaral, R. G., dos Santos, S. A., Andrade, L. N., Severino, P., & Carvalho, A. A. (2019). Natural products as treatment against cancer: A historical and current vision. Clinical Oncology (Royal College of Radiologist), 4(5), 1562.
Mellor, H. R., & Callaghan, R. (2008). Resistance to chemotherapy in cancer: A complex and integrated cellular response. Pharmacology, 81(4), 275–300.
Broxterman, H. J., Gotink, K. J., & Verheul, H. M. W. (2009). Understanding the causes of multidrug resistance in cancer: A comparison of doxorubicin and sunitinib. Drug Resist. Updat., 12(4–5), 114–126.
David-Beabes, G. L., Overman, M. J., Petrofski, J. A., Campbell, P. A., de Marzo, A. M., & Nelson, W. G. (2000). Doxorubicin-resistant variants of human prostate cancer cell lines DU 145, PC-3, PPC-1, and TSU-PR1: Characterization of biochemical determinants of antineoplastic drug sensitivity. International Journal of Oncology, 17(6), 1077–1163.
Frederiksen, L. J., Siemens, D. R., Heaton, J. P., Maxwell, L. R., Adams, M. A., & Graham, C. H. (2003). Hypoxia induced resistance to doxorubicin in prostate cancer cells is inhibited by low concentrations of glyceryl trinitrate. Journal of Urology, 170(3), 1003–1007.
Gao, M., Guo, L., Wang, H., Huang, J., Han, F., Xiang, S., & Wang, J. (2020). Orphan nuclear receptor RORγ confers doxorubicin resistance in prostate cancer. Cell Biology International., 44(10), 2170–2176.
Grunwald, V., DeGraffenried, L., Russel, D., Friedrichs, W. E., Ray, R. B., & Hidalgo, M. (2002). Inhibitors of mTOR reverse doxorubicin resistance conferred by PTEN status in prostate cancer cells. Cancer Research, 62(21), 6141–6145.
Replogle-Schwab, T. S., Schwab, E. D., & Pienta, K. J. (1997). Development of doxorubicin resistant rat prostate cancer cell lines. Anticancer Research, 17(6D), 4535–4538.
Zhao, W., Ning, L., Wang, L., Ouyang, T., Qi, L., Yang, R., & Wu, Y. (2021). miR-21 inhibition reverses doxorubicin-resistance and inhibits PC3 human prostate cancer cells proliferation. Andrologia, 53(5), e14016.
Ohya, S., Kajikuri, J., Endo, K., Kito, H., & Matsui, M. (2021). KCa1. 1 K+ Channel Inhibition overcomes resistance to antiandrogens and doxorubicin in a human prostate cancer LNCaP spheroid model. International Journal of Molecular Sciences, 22(24), 13553.
Aniogo, E. C., George, B. P., & Abrahamse, H. (2020). Plant-Based Compounds as Alternative Adjuvant Therapy for Multidrug-Resistant Cancer. Phytomedicine (pp. 7–12). CRC Press.
Ng, C. X., Affendi, M. M., Chong, P. P., & Lee, S. H. (2022). The potential of plant-derived extracts and compounds to augment anticancer effects of chemotherapeutic drugs. Nutrition and Cancer, 74(9), 3058–3076.
Padhi, M., & Mahapatra, S. (2013). Boswellia serrata: A review of its traditional uses, phytochemistry and pharmacology. International Review of Biophysical Chemistry (IREBIC), 4, 74–83.
Yousef, J. M. (2011). Identifying frankincense impact by biochemical analysis and histological examination on rats. Saudi Journal of Biological Sciences, 18(2), 189–194.
Gupta, I., Parihar, A., Malhotra, P., Gupta, S., Lüdtke, R., Safayhi, H., & Ammon, H. P. (2001). Effects of gum resin of Boswellia serrata in patients with chronic colitis. Planta Medica., 67(05), 391–395.
Tilahun, M., Muys, B., Mathijs, E., Kleinn, C., Olschewski, R., & Gebrehiwot, K. (2011). Frankincense yield assessment and modeling in closed and grazed Boswellia papyrifera woodlands of Tigray, Northern Ethiopia. Journal of Arid Environments, 75(8), 695–702.
Chhetri, B. K., Awadh Ali, N. A., & Setzer, W. N. (2015). A survey of chemical compositions and biological activities of Yemeni aromatic medicinal plants. Medicines, 2(2), 67–92.
Shah, B. A., Qazi, G. N., & Taneja, S. C. (2009). Boswellic acids: A group of medicinally important compounds. Natural Products Reports, 26(1), 72–89.
Liu, J.-J., Nilsson, A., Oredsson, S., Badmaev, V., & Duan, R.-D. (2002). Keto-and acetyl-keto-boswellic acids inhibit proliferation and induce apoptosis in Hep G2 cells via a caspase-8 dependent pathway. International Journal of Molecular Medicine, 10(4), 501–505.
Takada, Y., Ichikawa, H., Badmaev, V., & Aggarwal, B. B. (2006). Acetyl-11-keto-β-boswellic acid potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis by suppressing NF-κB and NF-κB-regulated gene expression. The Journal of Immunology, 176(5), 3127–3140.
Yuan, H.-Q., Kong, F., Wang, X.-L., Young, C. Y. F., Hu, X.-Y., & Lou, H.-X. (2008). Inhibitory effect of acetyl-11-keto-β-boswellic acid on androgen receptor by interference of Sp1 binding activity in prostate cancer cells. Biochemical Pharmacology, 75(11), 2112–2121.
Liu, Y. Q., Wang, S. K., Xu, Q. Q., Yuan, H. Q., Guo, Y. X., Wang, Q., Kong, F., Lin, Z. M., Sun, D. Q., Wang, R. M., & Lou, H. X. (2019). Acetyl-11-keto-β-boswellic acid suppresses docetaxel-resistant prostate cancer cells in vitro and in vivo by blocking Akt and Stat3 signaling, thus suppressing chemoresistant stem cell-like properties. Acta Pharmacologica Sinica., 40(5), 689–698.
Pang, X., Yi, Z., Zhang, X., Sung, B., Qu, W., Lian, X., Aggarwal, B. B., & Liu, M. (2009). Acetyl-11-keto-β-boswellic acid inhibits prostate tumor growth by suppressing vascular endothelial growth factor receptor 2–mediated angiogenesis. Cancer Research., 69(14), 5893–5900.
Bahuguna, A., Khan, I., Bajpai, V. K., & Kang, S. C. (2017). “MTT assay to evaluate the cytotoxic potential of a drug. ||| Bangladesh Journal of Pharmacology|||, 12(2), 115–118.
Mani, S., & Swargiary, G. (2023). “In Vitro Cytotoxicity Analysis: MTT/XTT, Trypan Blue Exclusion. Animal Cell Culture: Principles and Practice (pp. 267–284). Springer.
Khan, I., Mahfooz, S., Saeed, M., Ahmad, I., & Ansari, I. A. (2021). “Andrographolide inhibits proliferation of colon cancer SW-480 cells via downregulating notch signaling pathway. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents), 21(4), 487–497.
Khan, F., Singh, V. K., Saeed, M., Kausar, M. A., & Ansari, I. A. (2019). “Carvacrol induced program cell death and cell cycle arrest in androgen-independent human prostate cancer cells via inhibition of notch signalling. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents), 19(13), 1588–1608.
Zhang, D., Li, K., Sun, C., Cao, G., Qi, Y., Lin, Z., Guo, Y., Liu, Z., Chen, Y., Liu, J., Cheng, G., Wang, P., Zhang, L., Zhang, J., Wen, J., Xu, D., Kong, F., & Zhao, S. (2018). Anti-cancer effects of Paris polyphylla ethanol extract by inducing cancer cell apoptosis and cycle arrest in prostate cancer cells. Current Urology., 11(3), 144–150.
Lodi, A., Saha, A., Lu, X., Wang, B., Sentandreu, E., Collins, M., Kolonin, M. G., DiGiovanni, J., & Tiziani, S. (2017). Combinatorial treatment with natural compounds in prostate cancer inhibits prostate tumor growth and leads to key modulations of cancer cell metabolism. npj Precision Oncology., 1(1), 18.
Roy, N. K., Parama, D., Banik, K., Bordoloi, D., Devi, A. K., Thakur, K. K., Padmavathi, G., Shakibaei, M., Fan, L., Sethi, G., & Kunnumakkara, A. B. (2019). An update on pharmacological potential of boswellic acids against chronic diseases. International Journal of Molecular Sciences, 20(17), 4101.
Li, K., Li, L., Wang, S., Li, X., Ma, T., Liu, D., Jing, Y., & Zhao, L. (2017). Design and synthesis of novel 2-substituted 11-keto-boswellic acid heterocyclic derivatives as anti-prostate cancer agents with Pin1 inhibition ability. European Journal of Medicinal Chemistry, 126, 910–919.
Pillai, P., Pooleri, G. K., & Nair, S. V. (2021). Role of testosterone levels on the combinatorial effect of Boswellia serrata extract and enzalutamide on androgen dependent LNCaP cells and in patient derived cells. Integrative Cancer Therapies, 20, 1534735421996824.
Park, Y. S., Lee, J. H., Harwalkar, J. A., Bondar, J., Safayhi, H., & Golubic, M. (2002). Acetyl-11-Keto-ß-Boswellic acid (Akba) is cytotoxic for meningioma cells and inhibits phosphorylation of the extracellular-signal regulated kinase 1 and 2. Eicosanoids and Other Bioactive Lipids in Cancer, Inflammation, and Radiation Injury, 5, 387–393.
Saraste, A., & Pulkki, K. (2000). Morphologic and biochemical hallmarks of apoptosis. Cardiovascular Research, 45(3), 528–537.
Zhang, B., Wang, D., Guo, F., & Xuan, C. (2015). Mitochondrial membrane potential and reactive oxygen species in cancer stem cells. Familial Cancer, 14, 19–23.
Tong, L., Chuang, C.-C., Wu, S., & Zuo, L. (2015). Reactive oxygen species in redox cancer therapy. Cancer Letters, 367(1), 18–25.
Kim, S. J., Kim, H. S., & Seo, Y. R. (2019). Understanding of ROS-inducing strategy in anticancer therapy. Oxidative Medicine and Cellular Longevity., 2019, 1–12.
Ciniglia, C., Pinto, G., Sansone, C., & Pollio, A. (2010). Acridine orange/Ethidium bromide double staining test: A simple In-vitro assay to detect apoptosis induced by phenolic compounds in plant cells. Allelopathy Journal, 26(2), 301–308.
Kasibhatla, S., Amarante-Mendes, G. P., Finucane, D., Brunner, T., Bossy-Wetzel, E., & Green, D. R. (2006). Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis. Csh Protocols, 2006(3), 4493.
Watanabe, M., Hitomi, M., van der Wee, K., Rothenberg, F., Fisher, S. A., Zucker, R., Svoboda, K. K., Goldsmith, E. C., Heiskanen, K. M., & Nieminen, A. L. (2002). The pros and cons of apoptosis assays for use in the study of cells, tissues, and organs. Microscopy and Microanalysis., 8(5), 375–391.
Jamwal, S., Kumar, P., Kakkar, V., Kumari, P., & Chahal, S. K. (2021). Protocols in apoptosis identification and affirmation. Clinical Perspectives and Targeted Therapies in Apoptosis (pp. 127–152). Elsevier.
Boice, A., & Bouchier-Hayes, L. (2020). Targeting apoptotic caspases in cancer. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1867(6), 118688.
Liu, J.-J., Nilsson, Å., Oredsson, S., Badmaev, V., Zhao, W.-Z., & Duan, R.-D. (2002). Boswellic acids trigger apoptosis via a pathway dependent on caspase-8 activation but independent on Fas/Fas ligand interaction in colon cancer HT-29 cells. Carcinogenesis, 23(12), 2087–2093.
Syrovets, T., Gschwend, J. E., Büchele, B., Laumonnier, Y., Zugmaier, W., Genze, F., & Simmet, T. (2005). Inhibition of IκB kinase activity by acetyl-boswellic acids promotes apoptosis in androgen-independent PC-3 prostate cancer cells in vitro and in vivo. Journal of Biological Chemistry., 280(7), 6170–6180.
Lu, M., Xia, L., Hua, H., & Jing, Y. (2008). Acetyl-keto-β-Boswellic acid induces apoptosis through a death receptor 5–mediated pathway in prostate cancer cells. Cancer Research, 68(4), 1180–1186.
Büchele, B., Zugmaier, W., Estrada, A., Genze, F., Syrovets, T., Paetz, C., Schneider, B., & Simmet, T. (2006). Characterization of 3α-acetyl-11-keto-α-boswellic acid, a pentacyclic triterpenoid inducing apoptosis in vitro and in vivo. Planta Medica., 72(14), 1285–1289.
Qurishi, Y., Hamid, A., Sharma, P. R., Wani, Z. A., Mondhe, D. M., Singh, S. K., Zargar, M. A., Andotra, S. S., Shah, B. A., Taneja, S. C., & Saxena, A. K. (2012). PARP cleavage and perturbance in mitochondrial membrane potential by 3-α-propionyloxy-β-boswellic acid results in cancer cell death and tumor regression in murine models. Future Oncology., 8(7), 867–881.
Pathania, A. S., Guru, S. K., Kumar, S., Kumar, A., Ahmad, M., Bhushan, S., Sharma, P. R., Mahajan, P., Shah, B. A., Sharma, S., Nargotra, A., Vishwakarma, R., Korkaya, H., & Malik, F. (2016). Interplay between cell cycle and autophagy induced by boswellic acid analog. Scientific Reports, 6(1), 33146.
Li, W., Liu, J., Fu, W., Zheng, X., Ren, L., Liu, S., Wang, J., Ji, T., & Du, G. (2018). 3-O-acetyl-11-keto-β-boswellic acid exerts anti-tumor effects in glioblastoma by arresting cell cycle at G2/M phase. Journal of Experimental & Clinical Cancer Research, 37, 1–15.
Jiang, X., Liu, Y., Zhang, G., Lin, S., Yuan, N., Wu, J., Yan, X., Ma, Y., & Ma, M. (2020). Acetyl-11-keto-β-boswellic acid inhibits precancerous breast lesion MCF-10AT cells via regulation of LINC00707/miR-206 that reduces estrogen receptor-α. Cancer Management and Research, 12, 2301–2314.
Wang, D., Ge, S., Bai, J., & Song, Y. (2018). Boswellic acid exerts potent anticancer effects in HCT-116 human colon cancer cells mediated via induction of apoptosis, cell cycle arrest, cell migration inhibition and inhibition of PI3K/AKT signalling pathway. Journal of B.U.ON., 23(2), 340–345.
Li, C., He, Q., Xu, Y., Lou, H., & Fan, P. (2022). Synthesis of 3-O-acetyl-11-keto-β-boswellic acid (AKBA)-derived amides and their mitochondria-targeted antitumor activities. ACS Omega, 7(11), 9853–9866.
Zhou, Z., Edil, B. H., & Li, M. (2023). Combination therapies for cancer: Challenges and opportunities. BMC Medicine, 21(1), 171.
Jin, H., Wang, L., & Bernards, R. (2023). Rational combinations of targeted cancer therapies: Background, advances and challenges. Nature Reviews Drug Discovery, 22(3), 213–234.
Amin, A. R. M. R., Kucuk, O., Khuri, F. R., & Shin, D. M. (2009). Perspectives for cancer prevention with natural compounds. Journal of Clinical Oncology, 27(16), 2712.
Cimino, S., Sortino, G., Favilla, V., Castelli, T., Madonia, M., Sansalone, S., Russo, G. I., & Morgia, G. (2012). Polyphenols: key issues involved in chemoprevention of prostate cancer. Oxidative Medicine and Cellular Longevity, 2012, 1–8.
Lv, M., Zhuang, X., Zhang, Q., Cheng, Y., Wu, D., Wang, X., & Qiao, T. (2021). Acetyl-11-keto-β-boswellic acid enhances the cisplatin sensitivity of non-small cell lung cancer cells through cell cycle arrest, apoptosis induction, and autophagy suppression via p21-dependent signaling pathway. Cell Biology and Toxicology, 37, 209–228.
Acknowledgements
The authors express their gratitude to DST-FIST for providing the necessary resources to carry out this research at Integral University. This manuscript has been assigned the communication number IU/R&D/2023-MCN0002087 by Integral University. Verma M also acknowledges the DST-INSPIRE Fellowship from the Department of Sciences and Technology, Government of India, for awarding the fellowship (SRF) for the smooth conduction of this work (DST-INSPIRE fellowship-IF180991). The Authors also acknowledge DST-FIST funding (SR/FST/LS-1/2017/13(C) to the Department of Biosciences, Integral University
Author information
Authors and Affiliations
Contributions
MV performed all the experiments and wrote the manuscript. SF helped in data summarization. IAA conceptualized, edited, analyzed, and finalized the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Verma, M., Fatima, S., Saeed, M. et al. Anti-proliferative, Pro-apoptotic, and Chemosensitizing Potential of 3-Acetyl-11-keto-β-boswellic Acid (AKBA) Against Prostate Cancer Cells. Mol Biotechnol (2024). https://doi.org/10.1007/s12033-024-01089-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12033-024-01089-7