Abstract
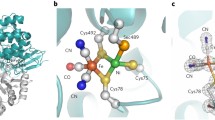
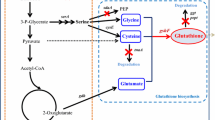
Glutathione peroxidase (GPx) is an important antioxidant enzyme. Selenocysteine (Sec)-containing GPxs (Sec-GPxs) are usually superior to their conventional cysteine-containing counterparts (Cys-GPxs), which make up the majority of the natural GPxs but display unsuitable activity and stability for industrial applications. This study first heterologously expressed and characterized a Cys-GPx from Lactococcus lactis (LlGPx), systematically exchanged all the three Cys to Sec and introduced an extra Sec. The results showed that the insertion of Sec at the active site could effectively increase the enzyme activity and confer a lower optimal pH value on the mutants. The double mutant C36U/L157U increased by 2.65 times (5.12 U/mg). The thermal stability of the C81U mutant was significantly improved. These results suggest that site-directed Sec incorporation can effectively improve the enzymatic properties of LlGPx, which may be also used for the protein engineering of other industrial enzymes containing catalytic or other functional cysteine residues.




Similar content being viewed by others
Data Availability
Applicable.
Peer Review
Externally peer-reviewed.
Consent to Publish
Not applicable.
References
Moosmayer, D., Hilpmann, A., Hoffmann, J., Schnirch, L., Zimmermann, K., Badock, V., Furst, L., Eaton, J. K., Viswanathan, V. S., Schreiber, S. L., Gradl, S., & Hillig, R. C. (2021). Crystal structures of the selenoprotein glutathione peroxidase 4 in its apo form and in complex with the covalently bound inhibitor ML162. Acta Crystallographica Section D Structural Biology, 77, 237–248. https://doi.org/10.1107/S2059798320016125
Dimastrogiovanni, D., Anselmi, M., Miele, A. E., Boumis, G., Petersson, L., Angelucci, F., Nola, A. D., Brunori, M., & Bellelli, A. (2010). Combining crystallography and molecular dynamics: The case of Schistosoma mansoni phospholipid glutathione peroxidase. Proteins, 78, 259–270. https://doi.org/10.1002/prot.22536
Toppo, S., Vanin, S., Bosello, V., & Tosatto, S. C. (2008). Evolutionary and structural insights into the multifaceted glutathione peroxidase (GPx) super family. Antioxidants and Redox Signaling, 10, 1501–1514. https://doi.org/10.1089/ars.2008.2057
Brigelius-Flohe, R., & Maiorino, M. (2013). Glutathione peroxidases. Biochimica Biophysica Acta, 1830, 3289–3303. https://doi.org/10.1016/j.bbagen.2012.11.020
Tosatto, S. C., Bosello, V., Fogolari, F., Mauri, P., Roveri, A., Toppo, S., Flohe, L., Ursini, F., & Maiorino, M. (2008). The catalytic site of glutathione peroxidases. Antioxidants and Redox Signaling, 10, 1515–1526. https://doi.org/10.1089/ars.2008.2055
Johansson, L., Gafvelin, G., & Arner, E. S. (2005). Selenocysteine in proteins—Properties and biotechnological use. Biochimica Biophysica Acta, 1726, 1–13. https://doi.org/10.1016/j.bbagen.2005.05.010
Rocher, C., Lalanne, J. L., & Chaudiere, J. (1992). Purification and properties of a recombinant sulfur analog of murine selenium-glutathione peroxidase. European Journal of Biochemistry, 205, 955–960. https://doi.org/10.1111/j.1432-1033.1992.tb16862.x
Maiorino, M., Aumann, K. D., Brigelius-Flohe, R., Doria, D., van den Heuvel, J., McCarthy, J., Roveri, A., Ursini, F., & Flohe, L. (1995). Probing the presumed catalytic triad of selenium-containing peroxidases by mutational analysis of phospholipid hydroperoxide glutathione peroxidase (PHGPx). Biological Chemistry Hoppe Seyler, 376, 651–660. https://doi.org/10.1515/bchm3.1995.376.11.651
Bunaciu, A. A., Danet, A. F., Fleschin, S., & Aboul-Enein, H. Y. (2016). Recent applications for in vitro antioxidant activity assay. Critical Reviews in Analytical Chemistry, 46, 389–399. https://doi.org/10.1080/10408347.2015.1101369
Edmondson, D. E. (2014). Hydrogen peroxide produced by mitochondrial monoamine oxidase catalysis: Biological implications. Current Pharmaceutical Design, 20, 155–160. https://doi.org/10.2174/13816128113190990406
Pan, T., Liu, Y., Si, C., Bai, Y., Qiao, S., Zhao, L., Xu, J., Dong, Z., Luo, Q., & Liu, J. (2017). Construction of ATP-switched allosteric antioxidant selenoenzyme. ACS Catalysis, 7, 1875–1879. https://doi.org/10.1021/acscatal.6b03274
Cheng, Q., & Arner, E. S. (2017). Selenocysteine insertion at a predefined UAG codon in a release factor 1 (RF1)-depleted Escherichia coli host strain bypasses species barriers in recombinant selenoprotein translation. Journal of Biological Chemistry, 292, 5476–5487. https://doi.org/10.1074/jbc.M117.776310
Isaacs, F. J., Carr, P. A., Wang, H. H., Lajoie, M. J., Sterling, B., Kraal, L., Tolonen, A. C., Gianoulis, T. A., Goodman, D. B., Reppas, N. B., Emig, C. J., Bang, D., Hwang, S. J., Jewett, M. C., Jacobson, J. M., & Church, G. M. (2011). Precise manipulation of chromosomes in vivo enables genome-wide codon replacement. Science, 333, 348–353. https://doi.org/10.1126/science.1205822
Lajoie, M. J., Rovner, A. J., Goodman, D. B., Aerni, H. R., Haimovich, A. D., Kuznetsov, G., Mercer, J. A., Wang, H. H., Carr, P. A., Mosberg, J. A., Rohland, N., Schultz, P. G., Jacobson, J. M., Rinehart, J., Church, G. M., & Isaacs, F. J. (2013). Genomically recoded organisms expand biological functions. Science, 342, 357–360. https://doi.org/10.1126/science.1241459
Flohe, L., & Brand, I. (1970). Some hints to avoid pitfalls in quantitative determination of glutathione peroxidase (EC 1.11.1.9). Zeitschrift für Klinische Chemie und Klinische Biochemie, 8, 156–161. https://doi.org/10.1515/cclm.1970.8.2.156
Zhang, W. J., He, Y. X., Yang, Z., Yu, J., Chen, Y., & Zhou, C. Z. (2008). Crystal structure of glutathione-dependent phospholipid peroxidase Hyr1 from the yeast Saccharomyces cerevisiae. Proteins, 73, 1058–1062. https://doi.org/10.1002/prot.22220
Turanov, A. A., Lobanov, A. V., Hatfield, D. L., & Gladyshev, V. N. (2013). UGA codon position-dependent incorporation of selenocysteine into mammalian selenoproteins. Nucleic Acids Research, 41, 6952–6959. https://doi.org/10.1093/nar/gkt409
DallaTiezza, M., Bickelhaupt, F. M., Flohe, L., Maiorino, M., Ursini, F., & Orian, L. (2020). A dual attack on the peroxide bond. The common principle of peroxidatic cysteine or selenocysteine residues. Redox Biology, 34, 101540. https://doi.org/10.1016/j.redox.2020.101540
Toppo, S., Flohe, L., Ursini, F., Vanin, S., & Maiorino, M. (2009). Catalytic mechanisms and specificities of glutathione peroxidases: Variations of a basic scheme. Biochimica Biophysica Acta, 1790, 1486–1500. https://doi.org/10.1016/j.bbagen.2009.04.007
Brigelius-Flohe, R., & Flohe, L. (2011). Basic principles and emerging concepts in the redox control of transcription factors. Antioxidants and Redox Signaling, 15, 2335–2381. https://doi.org/10.1089/ars.2010.3534
Zeida, A., Trujillo, M., Ferrer-Sueta, G., Denicola, A., Estrin, D. A., & Radi, R. (2019). Catalysis of peroxide reduction by fast reacting protein thiols. Chemical Reviews, 119, 10829–10855. https://doi.org/10.1021/acs.chemrev.9b00371
Epp, O., Ladenstein, R., & Wendel, A. (1983). The refined structure of the selenoenzyme glutathione peroxidase at 0.2-nm resolution. European Journal of Biochemistry, 133, 51–69. https://doi.org/10.1111/j.1432-1033.1983.tb07429.x
Ladenstein, R., Epp, O., Bartels, K., Jones, A., Huber, R., & Wendel, A. (1979). Structure analysis and molecular model of the selenoenzyme glutathione peroxidase at 2.8 A resolution. Journal of Molecular Biology, 134, 199–218. https://doi.org/10.1016/0022-2836(79)90032-9
Ferrer-Sueta, G., Manta, B., Botti, H., Radi, R., Trujillo, M., & Denicola, A. (2011). Factors affecting protein thiol reactivity and specificity in peroxide reduction. Chemical Research in Toxicology, 24, 434–450. https://doi.org/10.1021/tx100413v
Hildebrandt, T., Knuesting, J., Berndt, C., Morgan, B., & Scheibe, R. (2015). Cytosolic thiol switches regulating basic cellular functions: GAPDH as an information hub? Biological Chemistry, 396, 523–537. https://doi.org/10.1515/hsz-2014-0295
Funding
This work was supported by the National Natural Science Foundation of China General Project (31972050).
Author information
Authors and Affiliations
Contributions
Conception/design of study—CHW, YHF; data acquisition—YHF, YMZ; data analysis/interpretation—YHF, YMZ, SYY, JJP, CXL, CHW; drafting manuscript—YHF, CHW; critical revision of manuscript—YHF, CHW; final approval and accountability—YHF, CHW. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical Approval
Not applicable.
Informed Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fang, YH., Zhang, YM., Yue, SY. et al. Improving Catalytic Activity, Acid-Tolerance, and Thermal Stability of Glutathione Peroxidase by Systematic Site-Directed Selenocysteine Incorporation. Mol Biotechnol 65, 1644–1652 (2023). https://doi.org/10.1007/s12033-023-00682-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-00682-6