Abstract
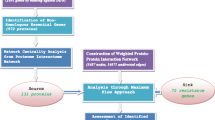
The reconstruction and analysis of the protein–protein interaction (PPI) network is a powerful approach to understand the complex biological and molecular functions in normal and disease states of the cell. The interactome of most organisms is largely unidentified except some model organisms. The current study focused on the construction of PPI network for the human pathogen Mycobacterium tuberculosis (MTB)-resistant strain XDR1219 using computational methods. In this work, a bioinformatics approach was employed to reveal potential drug targets. The pipeline adopted the combination of an extensive integrated network analysis that led to identify 22 key proteins involved in drug resistance, resistant metabolic pathways, virulence, pathogenesis and persistency of the infection. The MTB XDR1219 interactome consists of 11,383 non-redundant PPIs among 1499 proteins covering 38% of the entire MTB XDR1219 proteome. The overall quality of the network was assessed and topological parameters of the PPI were calculated. The predicted interactions were functionally annotated and their relevance was assessed with the functional similarity. The study attempts to present the interactome of previously unidentified MTB XDR1219 and revealed potential drug targets that can be further explored by scientific community.






Similar content being viewed by others
References
Wang, J., Zhao, Y., Gong, W., Liu, Y., Wang, M., Huang, X., & Tan, J. (2021). EDLMFC: An ensemble deep learning framework with multi-scale features combination for ncRNA–protein interaction prediction. BMC Bioinformatics, 22, 1–19.
Zeng, M., Zhang, F., Wu, F.-X., Li, Y., Wang, J., & Li, M. (2019). Protein–protein interaction site prediction through combining local and global features with deep neural networks. Bioinformatics, 36, 1114–1120.
Peng, X., Wang, J., Peng, W., Wu, F.-X., & Pan, Y. (2016). Protein–protein interactions: Detection, reliability assessment and applications. Briefings in Bioinformatics, 18, 798–819.
Verma, H., Nagar, S., Vohra, S., Pandey, S., Lal, D., Negi, R. K., Lal, R., & Rawat, C. D. (2021). Genome analyses of 174 strains of Mycobacterium tuberculosis provide insight into the evolution of drug resistance and reveal potential drug targets. Microbial Genomics, 7, mgen000542.
Global tuberculosis report 2020, Geneva: World Health Organization, (2020). Licence: CC BY-NC-SA 3.0 IGO.
Yuan, T., Werman, J. M., & Sampson, N. S. (2021). The pursuit of mechanism of action: Uncovering drug complexity in TB drug discovery. RSC Chemical Biology, 2, 423–440.
Agamah, F. E., Mazandu, G. K., Hassan, R., Bope, C. D., Thomford, N. E., Ghansah, A., & Chimusa, E. R. (2020). Computational/in silico methods in drug target and lead prediction. Briefings in Bioinformatics, 21, 1663–1675.
Gupta, S. K., Osmanoglu, Ö., Srivastava, M., Bencúrová, E., & Dandekar, T. (2020). Pathogen and host-pathogen protein interactions provide a key to identify novel drug targets. In O. Wolkenhauer (Ed.), Systems medicine: Integrative, qualitative and computational approaches (Vol. 2, pp. 543–553). Academic Press.
Li, X., Li, W., Zeng, M., Zheng, R., & Li, M. (2019). Network-based methods for predicting essential genes or proteins: A survey. Briefings in Bioinformatics, 21, 566–583.
Banerjee, U., Sankar, S., Singh, A., & Chandra, N. (2020). A multi-pronged computational pipeline for prioritizing drug target strategies for latent tuberculosis. Frontiers in Chemistry, 8, 1193.
Zhang, Y., Zhang, X., Zhao, Z., Zheng, Y., Xiao, Z., & Li, F. (2019). Integrated bioinformatics analysis and validation revealed potential immune-regulatory miR-892b, miR-199b-5p and miR-582–5p as diagnostic biomarkers in active tuberculosis. Microbial Pathogenesis, 134, 103563.
Borham, M., Oreiby, A., El-Gedawy, A., Hegazy, Y., Hemedan, A., & Al-Gaabary, M. (2021). Abattoir survey of bovine tuberculosis in tanta, centre of the Nile delta, with in silico analysis of gene mutations and protein–protein interactions of the involved mycobacteria. Transboundary and Emerging Diseases. https://doi.org/10.1111/tbed.14001
Yu, X., Feng, J., Huang, L., Gao, H., Liu, J., Bai, S., Wu, B., & Xie, J. (2019). Molecular basis underlying host immunity subversion by Mycobacterium tuberculosis PE/PPE family molecules. DNA and Cell Biology, 38, 1178–1187.
Szklarczyk, D., Morris, J. H., Cook, H., Kuhn, M., Wyder, S., Simonovic, M., Santos, A., Doncheva, N. T., Roth, A., & Bork, P. (2016). The STRING database in 2017: Quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Research, 45, D362–D368.
Consortium, U. (2019). UniProt: A worldwide hub of protein knowledge. Nucleic Acids Research, 47, D506–D515.
Luo, H., Lin, Y., Gao, F., Zhang, C.-T., & Zhang, R. (2014). DEG 10, an update of the database of essential genes that includes both protein-coding genes and noncoding genomic elements. Nucleic Acids Research, 42, D574–D580.
Hernández-Salmerón, J. E., & Moreno-Hagelsieb, G. (2020). Progress in quickly finding orthologs as reciprocal best hits: Comparing blast, last, diamond and MMseqs2. BMC Genomics, 21, 1–9.
Gurumayum, S., Jiang, P., Hao, X., Campos, T. L., Young, N. D., Korhonen, P. K., Gasser, R. B., Bork, P., Zhao, X.-M., & He, L.-J. (2021). OGEE v3: Online GEne Essentiality database with increased coverage of organisms and human cell lines. Nucleic Acids Research, 49, D998–D1003.
Alcock, B. P., Raphenya, A. R., Lau, T. T., Tsang, K. K., Bouchard, M., Edalatmand, A., Huynh, W., Nguyen, A.-L.V., Cheng, A. A., & Liu, S. (2020). CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Research, 48, D517–D525.
Uddin, R., Tariq, S. S., Azam, S. S., Wadood, A., & Moin, S. T. (2017). Identification of histone deacetylase (HDAC) as a drug target against MRSA via interolog method of protein-protein interaction prediction. European Journal of Pharmaceutical Sciences, 106, 198–211.
Mei, S., Flemington, E. K., & Zhang, K. (2018). Transferring knowledge of bacterial protein interaction networks to predict pathogen targeted human genes and immune signaling pathways: A case study on M. tuberculosis. BMC Genomics, 19, 1–21.
Kohl, M., Wiese, S., & Warscheid, B. (2011). Cytoscape: Software for visualization and analysis of biological networks. In M., Hamacher, M., Eisenacher, & C. Stephan (Eds.), Data mining in proteomics. Methods in molecular biology (Methods and protocols) (Vol. 696, pp. 291–303). Humana Press.
Huerta-Cepas, J., Szklarczyk, D., Forslund, K., Cook, H., Heller, D., Walter, M. C., Rattei, T., Mende, D. R., Sunagawa, S., Kuhn, M., Jensen, L. J., von Mering, C., & Bork, P. (2015). eggNOG 4.5: A hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucleic Acids Research, 44, D286–D293.
Kanehisa, M., Furumichi, M., Tanabe, M., Sato, Y., & Morishima, K. (2017). KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Research, 45, D353–D361.
Yu, N. Y., Wagner, J. R., Laird, M. R., Melli, G., Rey, S., Lo, R., Dao, P., Sahinalp, S. C., Ester, M., & Foster, L. J. (2010). PSORTb 3.0: Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics, 26, 1608–1615.
Assenov, Y., Ramírez, F., Schelhorn, S.-E., Lengauer, T., & Albrecht, M. (2008). Computing topological parameters of biological networks. Bioinformatics, 24, 282–284.
Chin, C.-H., Chen, S.-H., Wu, H.-H., Ho, C.-W., Ko, M.-T., & Lin, C.-Y. (2014). cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Systems Biology, 8, 1–7.
Bader, G. D., & Hogue, C. W. (2003). An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics, 4, 1–27.
Zhao, C., & Wang, Z. (2018). GOGO: An improved algorithm to measure the semantic similarity between gene ontology terms. Science and Reports, 8, 1–10.
Gupta, S. K., Srivastava, M., Osmanoglu, Ö., & Dandekar, T. (2020). Genome-wide inference of the Camponotus floridanus protein-protein interaction network using homologous mapping and interacting domain profile pairs. Science and Reports, 10, 1–12.
Ning, K., Ng, H. K., Srihari, S., Leong, H. W., & Nesvizhskii, A. I. (2010). Examination of the relationship between essential genes in PPI network and hub proteins in reverse nearest neighbor topology. BMC Bioinformatics, 11, 1–14.
Wishart, D. S., Feunang, Y. D., Guo, A. C., Lo, E. J., Marcu, A., Grant, J. R., Sajed, T., Johnson, D., Li, C., & Sayeeda, Z. (2018). DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Research, 46, D1074–D1082.
Mahajan, G., & Mande, S. C. (2017). Using structural knowledge in the protein data bank to inform the search for potential host-microbe protein interactions in sequence space: Application to Mycobacterium tuberculosis. BMC Bioinformatics, 18, 1–14.
Zhou, H., Gao, S., Nguyen, N. N., Fan, M., Jin, J., Liu, B., Zhao, L., Xiong, G., Tan, M., Li, S., & Wong, L. (2014). Stringent homology-based prediction of H. sapiens-M. tuberculosis H37Rv protein-protein interactions. Biology Direct, 9, 1–30.
Han, Y.-C., Song, J.-M., Wang, L., Shu, C.-C., Guo, J., & Chen, L.-L. (2016). Prediction and characterization of protein-protein interaction network in Bacillus licheniformis WX-02. Science and Reports, 6, 1–11.
Shekarappa, S. B., Kandagalla, S., Gollapalli, P., Rudresh, B. B., Hanumanthappa, T., & Hanumanthappa, M. (2017). Topology of protein–protein interaction network and edge reduction co-efficiency in VEGF signaling of breast cancer. Network Modeling Analysis in Health Informatics and Bioinformatics, 6, 1–11.
Pang, E., Hao, Y., Sun, Y., & Lin, K. (2016). Differential variation patterns between hubs and bottlenecks in human protein-protein interaction networks. BMC Evolutionary Biology, 16, 1–9.
Thakur, Z., Dharra, R., Saini, V., Kumar, A., & Mehta, P. K. (2017). Insights from the protein-protein interaction network analysis of Mycobacterium tuberculosis toxin-antitoxin systems. Bioinformation, 13, 380–387.
Gabriele, T., Ivan, B., Fausto, S., Carlo, L., & Giovanni, S. (2017). Creating, generating and comparing random network models with NetworkRandomizer. F1000Research, 5, 1–15.
Bollobás, B. (1998). Random graphs. In Ravi Vakil (Ed.), Modern graph theory, Graduate Texts in Mathematics (Vol 184, pp. 215–252). New York, NY: Springer.
Watts, D. J., & Strogatz, S. H. (1998). Collective dynamics of ‘small-world’ networks. Nature, 393, 440–442.
Barabási, A.-L., & Albert, R. (1999). Emergence of scaling in random networks. Science, 286, 509–512.
Li, G., Li, M., Wang, J., Wu, J., Wu, F.-X., & Pan, Y. (2016). Predicting essential proteins based on subcellular localization, orthology and PPI networks. BMC Bioinformatics, 17, 571–581.
Miryala, S. K., Anbarasu, A., & Ramaiah, S. (2021). Gene interaction network approach to elucidate the multidrug resistance mechanisms in the pathogenic bacterial strain Proteus mirabilis. Journal of Cellular Physiology, 236, 468–479.
Yu, H., Kim, P. M., Sprecher, E., Trifonov, V., & Gerstein, M. (2007). The importance of bottlenecks in protein networks: Correlation with gene essentiality and expression dynamics. PLoS Computational Biology, 3, e59.
Radusky, L., Defelipe, L. A., Lanzarotti, E., Luque, J., Barril, X., Marti, M. A., & Turjanski, A. G. (2014). TuberQ: A Mycobacterium tuberculosis protein druggability database. Database, 2014, 1–10.
Wang, L., Sun, Y., Zhao, L., Xu, X., Huang, L., Qin, Y., Su, Y., Zhang, J., & Yan, Q. (2019). Dual RNA-seq uncovers the immune response of Larimichthys crocea to the secY gene of Pseudomonas plecoglossicida from the perspective of host-pathogen interactions. Fish & Shellfish Immunology, 93, 949–957.
Datta, P., Dasgupta, A., Singh, A. K., Mukherjee, P., Kundu, M., & Basu, J. (2006). Interaction between FtsW and penicillin-binding protein 3 (PBP3) directs PBP3 to mid-cell, controls cell septation and mediates the formation of a trimeric complex involving FtsZ, FtsW and PBP3 in mycobacteria. Molecular Microbiology, 62, 1655–1673.
Katiyar, A., Singh, H., & Azad, K. K. (2018). Identification of missing carbon fixation enzymes as potential drug targets in Mycobacterium tuberculosis. Journal of Integrative Bioinformatics, 15, 1–15.
Sitote, T. M., & Gakkhar, S. (2017). Structural analysis of protein translocase subunit SecY from Mycobacterium tuberculosis H37Rv: A potential target for anti-tuberculosis drug discovery. International Journal of Computational Biology and Drug Design, 10, 374–386.
Huang, J., Huang, L., Cai, K., Xu, Z., Tao, S.-C., & Wang, J.-F. (2019). RIBOi: A database for ribosome-interacting proteins. Acta Biochimica et Biophysica Sinica, 51, 441–443.
Singh, R., Dwivedi, S. P., Gaharwar, U. S., Meena, R., Rajamani, P., & Prasad, T. (2020). Recent updates on drug resistance in Mycobacterium tuberculosis. Journal of Applied Microbiology, 128, 1547–1567.
Shetye, G. S., Franzblau, S. G., & Cho, S. (2020). New tuberculosis drug targets, their inhibitors, and potential therapeutic impact. Translational Research, 220, 68–97.
Stefan, M. A., Velazquez, G. M., & Garcia, G. A. (2020). High-throughput screening to discover inhibitors of the CarD· RNA polymerase protein–protein interaction in Mycobacterium tuberculosis. Science and Reports, 10, 1–12.
Yu, X., Lai, S., Chen, H., & Chen, M. (2020). Protein–protein interaction network with machine learning models and multiomics data reveal potential neurodegenerative disease-related proteins. Human Molecular Genetics, 29, 1378–1387.
Wang, Y., You, Z., Li, L., & Chen, Z. (2020). A survey of current trends in computational predictions of protein-protein interactions. Frontiers of Computer Science, 14, 1–12.
Zhang, L., Yu, G., Xia, D., & Wang, J. (2019). Protein–protein interactions prediction based on ensemble deep neural networks. Neurocomputing, 324, 10–19.
Szilagyi, A., Grimm, V., Arakaki, A. K., & Skolnick, J. (2005). Prediction of physical protein–protein interactions. Physical Biology, 2, S1.
Chowdhury, S., Happonen, L., Khakzad, H., Malmström, L., & Malmström, J. (2020). Structural proteomics, electron cryo-microscopy and structural modeling approaches in bacteria–human protein interactions. Medical Microbiology and Immunology, 209, 265–275.
Uddin, R., & Azam, S. S. (2019). Identification of glucosyl-3-phosphoglycerate phosphatase as a novel drug target against resistant strain of Mycobacterium tuberculosis (XDR1219) by using comparative metabolic pathway approach. Computational Biology and Chemistry, 79, 91–102.
Singh, P., Rao, R. N., Reddy, J. R. C., Prasad, R., Kotturu, S. K., Ghosh, S., & Mukhopadhyay, S. (2016). PE11, a PE/PPE family protein of Mycobacterium tuberculosis is involved in cell wall remodeling and virulence. Science and Reports, 6, 1–16.
Soni, D. K., Dubey, S. K., & Bhatnagar, R. (2020). ATP-binding cassette (ABC) import systems of Mycobacterium tuberculosis: Target for drug and vaccine development. Emerging Microbes & Infections, 9, 207–220.
Gupta, S., Kumar, A., Singh, K., Kumari, R., Sharma, A., Singh, R. K., Pandey, S. K., & Anupurba, S. (2020). Rv1273c, an ABC transporter of Mycobacterium tuberculosis promotes mycobacterial intracellular survival within macrophages via modulating the host cell immune response. International Journal of Biological Macromolecules, 142, 320–331.
Kling, A., Lukat, P., Almeida, D. V., Bauer, A., Fontaine, E., Sordello, S., Zaburannyi, N., Herrmann, J., Wenzel, S. C., & König, C. (2015). Targeting DnaN for tuberculosis therapy using novel griselimycins. Science, 348, 1106–1112.
van Eijk, E., Wittekoek, B., Kuijper, E. J., & Smits, W. K. (2017). DNA replication proteins as potential targets for antimicrobials in drug-resistant bacterial pathogens. Journal of Antimicrobial Chemotherapy, 72, 1275–1284.
Zhan, L., Wang, J., Wang, L., & Qin, C. (2020). The correlation of drug resistance and virulence in Mycobacterium tuberculosis. Biosafety and Health, 2, 18–24.
Ali, A., Hasan, Z., McNerney, R., Mallard, K., Hill-Cawthorne, G., Coll, F., Nair, M., Pain, A., Clark, T. G., & Hasan, R. (2015). Whole genome sequencing based characterization of extensively drug-resistant Mycobacterium tuberculosis isolates from Pakistan. PLoS One, 10, e0117771.
Nikolay, R., van den Bruck, D., Achenbach, J., & Nierhaus, K. H. (2015). Ribosomal proteins: Role in ribosomal functions. In eLS (Vol. 2015, pp. 1–12), John Wiley & Sons, Ltd: Chichester.
Kerns, P. W., Ackhart, D. F., Basaraba, R. J., Leid, J. G., & Shirtliff, M. E. (2014). Mycobacterium tuberculosis pellicles express unique proteins recognized by the host humoral response. Pathogens and Disease, 70, 347–358.
Li, M., Gašparovič, H., Weng, X., Chen, S., Korduláková, J., & Jessen-Trefzer, C. (2020). The two-component locus MSMEG_0244/0246 together with MSMEG_0243 affects biofilm assembly in M. smegmatis correlating with changes in phosphatidylinositol mannosides acylation. Frontiers in Microbiology, 11, 1–15.
Manganelli, R. (2014). Sigma factors: Key molecules in Mycobacterium tuberculosis physiology and virulence. In G. F. Hatfull & W. R., Jacobs Jr. (Eds.), Molecular genetics of mycobacteria (pp. 135–160). ASM Press.
Swain, S. S., Sharma, D., Hussain, T., & Pati, S. (2020). Molecular mechanisms of underlying genetic factors and associated mutations for drug resistance in Mycobacterium tuberculosis. Emerging Microbes & Infections, 9, 1651–1663.
Pisu, D., Provvedi, R., Espinosa, D. M., Payan, J. B., Boldrin, F., Palù, G., Hernandez-Pando, R., & Manganelli, R. (2017). The alternative sigma factors SigE and SigB are involved in tolerance and persistence to antitubercular drugs. Antimicrobial Agents and Chemotherapy, 61, 1–11.
Abrahams, K. A., & Besra, G. S. (2021). Synthesis and recycling of the mycobacterial cell envelope. Current Opinion in Microbiology, 60, 58–65.
Edoo, Z., Iannazzo, L., Compain, F., Li de la Sierra Gallay, I., van Tilbeurgh, H., Fonvielle, M., Bouchet, F., Le Run, E., Mainardi, J. L., & Arthur, M. (2018). Synthesis of avibactam derivatives and activity on β-lactamases and peptidoglycan biosynthesis enzymes of mycobacteria. Chemistry—A European Journal, 24, 8081–8086.
Lu, Z., Wang, H., Zhang, A., Liu, X., Zhou, W., Yang, C., Guddat, L., Yang, H., Schofield, C. J., & Rao, Z. (2020). Structures of Mycobacterium tuberculosis penicillin-binding protein 3 in complex with five β-lactam antibiotics reveal mechanism of inactivation. Molecular Pharmacology, 97, 287–294.
Chen, Y., Xu, Y., Yang, S., Li, S., Ding, W., & Zhang, W. (2019). Deficiency of D-alanyl-D-alanine ligase A attenuated cell division and greatly altered the proteome of Mycobacterium smegmatis. MicrobiologyOpen, 8, e00819.
Yelamanchi, S. D., & Surolia, A. (2021). Targeting amino acid metabolism of Mycobacterium tuberculosis for developing inhibitors to curtail its survival. IUBMB Life, 73, 643–658.
Lee, J. J., Lim, J., Gao, S., Lawson, C. P., Odell, M., Raheem, S., Woo, J., Kang, S.-H., Kang, S.-S., & Jeon, B.-Y. (2018). Glutamate mediated metabolic neutralization mitigates propionate toxicity in intracellular Mycobacterium tuberculosis. Science and Reports, 8, 1–13.
Li, S., Su, Z., Zhang, C., Xu, Z., Chang, X., Zhu, J., Xiao, R., Li, L., & Zhou, R. (2018). Identification of drug target candidates of the swine pathogen Actinobacillus pleuropneumoniae by construction of protein–protein interaction network. Genes & Genomics, 40, 847–856.
Tan, M. F., Zou, G., Wei, Y., Liu, W. Q., Li, H. Q., Hu, Q., Zhang, L. S., & Zhou, R. (2020). Protein–protein interaction network and potential drug target candidates of Streptococcus suis. Journal of Applied Microbiology, 131, 658–670.
Funding
Funding was provided by Pakistan Science Foundation (Grant No. Med 431) and International Foundation for Science (Grant No. I-1-F-5378-2).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zahra, N.u.A., Jamil, F. & Uddin, R. Protein Integrated Network Analysis to Reveal Potential Drug Targets Against Extended Drug-Resistant Mycobacterium tuberculosis XDR1219. Mol Biotechnol 63, 1252–1267 (2021). https://doi.org/10.1007/s12033-021-00377-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-021-00377-w