Abstract
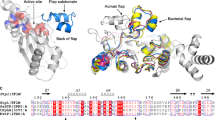
One of the most widespread pathogens worldwide is methicillin-resistant Staphylococcus aureus, a bacterium that provokes severe life-threatening illnesses both in hospitals and in the community. The principal challenge lies in the resistance of MRSA to current treatments, which encourages the study of different molecular targets that could be used to develop new drugs against this infectious agent. With this goal, a detailed characterization of shikimate kinase from this microorganism (SaSK) is described. The results showed that SaSK has a Km of 0.153 and 224 µM for shikimate and ATP, respectively, and a global reaction rate of 13.4 µmol/min/mg; it is suggested that SaSK utilizes the Bi–Bi Ping Pong reaction mechanism. Furthermore, the physicochemical data indicated that SaSK is an unstable, hydrophilic, and acidic protein. Finally, structural information showed that SaSK presented folding that is typical of its homologous counterparts and contains the typical domains of this family of proteins. Amino acids that have been shown to be important for SaSK protein function are conserved. Therefore, this study provides fundamental information that may aid in the design of inhibitors that could be used to develop new antibacterial agents.








Similar content being viewed by others
References
Stryjewski, M. E., & Corey, G. R. (2014). Methicillin-resistant Staphylococcus aureus: An evolving pathogen. Clinical Infectious Diseases, 58, S10–S19.
Kluytmans, J., Van Belkum, A., & Verbrugh, H. (1997). Nasal carriage of Staphylococcus aureus: Epidemiology, underlying mechanisms, and associated risks. Clinical Microbiology Reviews, 10, 505–520.
Wertheim, H. F., Melles, D. C., Vos, M. C., van Leeuwen, W., van Belkum, A., Verbrugh, H. A., & Nouwen, J. L. (2005). The role of nasal carriage in Staphylococcus aureus infections. The Lancet Infectious Diseases, 5, 751–762.
King, M. D., Humphrey, B. J., Wang, Y. F., Kourbatova, E. V., Ray, S. M., & Blumberg, H. M. (2006). Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Annals of Internal Medicine, 144, 309–317.
Drew, R. H. (2007). Emerging options for treatment of invasive, multidrug-resistant Staphylococcus aureus infections. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 27, 227–249.
Bentley, R., & Haslam, E. (1990). The shikimate pathway—a metabolic tree with many branche. Critical Reviews in Biochemistry and Molecular Biology, 25, 307–384.
Herrmann, K. M., & Weaver, L. M. (1999). The shikimate pathway. Annual Review of Plant Biology, 50, 473–503.
Dewick, P. M. (1995). The biosynthesis of shikimate metabolites. Natural Product Reports, 12, 101–133.
Kapnick, S. M., & Zhang, Y. (2008). New tuberculosis drug development: Targeting the shikimate pathway. Expert Opinion on Drug Discovery, 3, 565–577.
Griffin, H. G., & Gasson, M. J. (1995). The gene (aroK) encoding shikimate kinase I from Escherichia coli. DNA Sequence, 5, 195–197.
Yan, H., & Tsai, M. D. (1999). Nucleoside monophosphate kinases: Structure, mechanism, and substrate specificity. Advances in Enzymology and Related Areas of Molecular Biology: Mechanism of Enzyme Action, Part A, 73, 103–134.
Hartmann, M. D., Bourenkov, G. P., Oberschall, A., Strizhov, N., & Bartunik, H. D. (2006). Mechanism of phosphoryl transfer catalyzed by shikimate kinase from Mycobacterium tuberculosis. Journal of Molecular Biology, 364, 411–423.
Cheng, W.-C., Chang, Y.-N., & Wang, W.-C. (2005). Structural basis for shikimate-binding specificity of Helicobacter pylori shikimate kinase. Journal of Bacteriology, 187, 8156–8163.
Pereira, J. H., De Oliveira, J. S., Canduri, F., Dias, M. V., Palma, M. S., Basso, L. A., Santos, D. S., & De Azevedo, W. F. (2004). Structure of shikimate kinase from Mycobacterium tuberculosis reveals the binding of shikimic acid. Acta Crystallographica Section D: Biological Crystallography, 60, 2310–2319.
Sutton, K. A., Breen, J., MacDonald, U., Beanan, J. M., Olson, R., Russo, T. A., Schultz, L. W., & Umland, T. C. (2015). Structure of shikimate kinase, an in vivo essential metabolic enzyme in the nosocomial pathogen Acinetobacter baumannii, in complex with shikimate. Acta Crystallographica Section D: Biological Crystallography, 71, 1736–1744.
Cheng, W.-C., Chen, Y.-F., Wang, H.-J., Hsu, K.-C., Lin, S.-C., Chen, T.-J., Yang, J.-M., & Wang, W.-C. (2012). Structures of Helicobacter pylori shikimate kinase reveal a selective inhibitor-induced-fit mechanism. PLoS ONE, 7, e33481.
Romanowski, M. J., & Burley, S. K. (2002). Crystal structure of the Escherichia coli shikimate kinase I (AroK) that confers sensitivity to mecillinam. Proteins: Structure, Function, and Bioinformatics, 47, 558–562.
Krell, T., Coyle, J., Horsburgh, M., Coggins, J., & Lapthorn, A. (1997). Crystallization and preliminary X-ray crystallographic analysis of shikimate kinase from Erwinia chrysanthemi. Acta Crystallographica Section D, 53, 612–614.
Vonrhein, C., Schlauderer, G. J., & Schulz, G. E. (1995). Movie of the structural changes during a catalytic cycle of nucleoside monophosphate kinases. Structure, 3, 483–490.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Millar, G., Lewendon, A., Hunter, M., & Coggins, J. (1986). The cloning and expression of the aroL gene from Escherichia coli K12. Purification and complete amino acid sequence of shikimate kinase II, the aroL-gene product. Biochemical Journal, 237, 427–437.
Hanes, C. S. (1932). Studies on plant amylases: The effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochemical Journal, 26, 1406.
Laemmli, U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. nature. 227, 680.
Gasteiger, E., Gattiker, A., Hoogland, C., Ivanyi, I., Appel, R. D., & Bairoch, A. (2003). ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Research, 31, 3784–3788.
Gasteiger, E., Hoogland, C., Gattiker, A., Wilkins, M. R., Appel, R. D., & Bairoch, A. (2005). Protein identification and analysis tools on the ExPASy server (pp. 571–607). In: The proteomics protocols handbook. New York: Springer.
Walker, J. M. (2005). The proteomics protocols handbook. New York: Springer.
Best, R. B., Zhu, X., Shim, J., Lopes, P. E., Mittal, J., Feig, M., & MacKerell Jr, A. D. (2012). Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone ϕ, ψ and side-chain χ1 and χ2 dihedral angles. Journal of Chemical Theory and Computation, 8, 3257–3273.
Vanommeslaeghe, K., Hatcher, E., Acharya, C., Kundu, S., Zhong, S., Shim, J., Darian, E., Guvench, O., Lopes, P., & Vorobyov, I. (2010). CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. Journal of Computational Chemistry, 31, 671–690.
Bjelkmar, P., Larsson, P., Cuendet, M. A., Hess, B., & Lindahl, E. (2010). Implementation of the CHARMM force field in GROMACS: Analysis of protein stability effects from correction maps, virtual interaction sites, and water models. Journal of Chemical Theory and Computation, 6, 459–466.
Zoete, V., Cuendet, M. A., Grosdidier, A., & Michielin, O. (2011). SwissParam: A fast force field generation tool for small organic molecules. Journal of Computational Chemistry, 32, 2359–2368.
Berendsen, H. J., Postma, J. v., van Gunsteren, W. F., DiNola, A., & Haak, J. (1984). Molecular dynamics with coupling to an external bath. The Journal of Chemical Physics, 81, 3684–3690.
Pastor, R. W., Brooks, B. R., & Szabo, A. (1988). An analysis of the accuracy of Langevin and molecular dynamics algorithms. Molecular Physics, 65, 1409–1419.
Amadei, A., Linssen, A. B. M., & Berendsen, H. J. C. (1993). Essential dynamics of proteins. Proteins: Structure, Function, and Bioinformatics, 17, 412–425.
Mesentean, S., Fischer, S., & Smith, J. C. (2006). Analyzing large-scale structural change in proteins: Comparison of principal component projection and sammon mapping. Proteins: Structure, Function, and Bioinformatics, 64, 210–218.
Hess, B., Kutzner, C., Van Der Spoel, D., & Lindahl, E. (2008). GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. Journal of Chemical Theory and Computation, 4, 435–447.
Pauli, I., Caceres, R. A., & de Azevedo Jr, W. F. (2008). Molecular modeling and dynamics studies of Shikimate Kinase from Bacillus anthracis. Bioorganic & Medicinal Chemistry, 16, 8098–8108.
Oliveira, J. S., Pinto, C. A., Basso, L. A., & Santos, D. S. (2001). Cloning and overexpression in soluble form of functional shikimate kinase and 5-enolpyruvylshikimate 3-phosphate synthase enzymes from Mycobacterium tuberculosis. Protein Expression and Purification, 22, 430–435.
Arora, N., Banerjee, A. K., & Murty, U. (2010). In silico characterization of Shikimate Kinase of Shigella flexneri: a potential drug target. Interdisciplinary Sciences: Computational Life Sciences, 2, 280–290.
DeFeyter, R. C., & Pittard, J. (1986). Purification and properties of shikimate kinase II from Escherichia coli K-12. Journal of Bacteriology, 165, 331–333.
Gu, Y., Reshetnikova, L., Li, Y., Wu, Y., Yan, H., Singh, S., & Ji, X. (2002). Crystal structure of shikimate kinase from Mycobacterium tuberculosis reveals the dynamic role of the LID domain in catalysis. Journal of Molecular Biology, 319, 779–789.
Chen, K., Dou, J., Tang, S., Yang, Y., Wang, H., Fang, H., & Zhou, C. (2012). Deletion of the aroK gene is essential for high shikimic acid accumulation through the shikimate pathway in E. coli. Bioresource Technology, 119, 141–147.
Rosado, L. A., Vasconcelos, I. B., Palma, M. S., Frappier, V., Najmanovich, R. J., Santos, D. S., & Basso, L. A. (2013). The mode of action of recombinant Mycobacterium tuberculosis shikimate kinase: Kinetics and thermodynamics analyses. PLoS ONE, 8, e61918.
Segel, I. H. (1975). Enzyme kinetics: Behavior and analysis of rapid equilibrium and steady state enzyme systems. New York: Wiley
Krell, T., Maclean, J., Boam, D. J., Cooper, A., Resmini, M., Brocklehurst, K., Kelly, S. M., Price, N. C., Lapthorn, A. J., & Coggins, J. R. (2001). Biochemical and X-ray crystallographic studies on shikimate kinase: The important structural role of the P-loop lysine. Protein Science, 10, 1137–1149.
Guruprasad, K., Reddy, B. B., & Pandit, M. W. (1990). Correlation between stability of a protein and its dipeptide composition: A novel approach for predicting in vivo stability of a protein from its primary sequence. Protein Engineering, Design and Selection, 4, 155–161.
Kyte, J., & Doolittle, R. F. (1982). A simple method for displaying the hydropathic character of a protein. Journal of Molecular Biology, 157, 105–132.
Arora, N., Narasu, M., & Banerjee, A. (2016). Shikimate kinase of Yersinia pestis: A sequence, structural and functional analysis. International Journal of Biomedical Data Mining, 5, 2.
Abele, U., & Schulz, G. (1995). High-resolution structures of adenylate kinase from yeast ligated with inhibitor Ap5A, showing the pathway of phosphoryl transfer. Protein Science, 4, 1262–1271.
Larkin, M. A., Blackshields, G., Brown, N., Chenna, R., McGettigan, P. A., McWilliam, H., Valentin, F., Wallace, I. M., Wilm, A., & Lopez, R. (2007). Clustal W and Clustal X version 2.0. Bioinformatics., 23, 2947–2948.
Robert, X., & Gouet, P. (2014) Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Research. 42, W320–W324.
Blanco, B., Prado, V. n., Lence, E., Otero, J. M., Garcia-Doval, C., Van Raaij, M. J., Llamas-Saiz, A. L., Lamb, H., Hawkins, A. R., & González-Bello, C. n (2013). Mycobacterium tuberculosis shikimate kinase inhibitors: Design and simulation studies of the catalytic turnover. Journal of the American Chemical Society, 135, 12366–12376.
Dhaliwal, B., Nichols, C. E., Ren, J., Lockyer, M., Charles, I., Hawkins, A. R., & Stammers, D. K. (2004). Crystallographic studies of shikimate binding and induced conformational changes in Mycobacterium tuberculosis shikimate kinase. FEBS Letters, 574, 49–54.
Gan, J., Gu, Y., Li, Y., Yan, H., & Ji, X. (2006). Crystal structure of Mycobacterium tuberculosis shikimate kinase in complex with shikimic acid and an ATP analogue. Biochemistry, 45, 8539–8545.
Stierand, K., Maaß, P. C., & Rarey, M. (2006). Molecular complexes at a glance: Automated generation of two-dimensional complex diagrams. Bioinformatics., 22, 1710–1716.
Acknowledgements
C.A.D. and A.T.V. acknowledge Consejo Nacional de Ciencia y Tecnología (CONACyT) for Grants Nos. 258694 and 257848. CONACyT is also acknowledged for the fellowship granted to A.F.C. (No. 326106).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Favela-Candia, A., Téllez-Valencia, A., Campos-Almazán, M. et al. Biochemical, Kinetic, and Computational Structural Characterization of Shikimate Kinase from Methicillin-Resistant Staphylococcus aureus. Mol Biotechnol 61, 274–285 (2019). https://doi.org/10.1007/s12033-019-00159-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-019-00159-5