Abstract
Despite the high incidence of breast cancer in women worldwide, there are still great challenges in the treatment process. Mitochondria are highly dynamic organelles, and their dynamics involve cellular energy conversion, signal conduction and other processes. In recent years, an increasing number of studies have affirmed the dynamics of mitochondria as the basis for cancer progression and metastasis; that is, an imbalance between mitochondrial fission and fusion may lead to the progression and metastasis of breast cancer. Here, we review the latest insights into mitochondrial dynamics in the progression of breast cancer and emphasize the clinical value of mitochondrial dynamics in diagnosis and prognosis, as well as important advances in clinical research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of breast cancer has gradually increased at a rate of 0.5% per year since 2000 [1]. However, with a better understanding of cancer and the popularization of early breast cancer screening, such as mammography, breast color ultrasound and breast magnetic resonance screening, the mortality rate of breast cancer has decreased slightly in recent years. Early menarche, late menopause, family history, and old age are all high-risk factors for breast cancer [2]. Moreover, the occurrence of breast cancer is related to mutations in breast cancer susceptibility genes 1 and 2 (BRCA1/2). Breast cancer is a heterogeneous disease involving genetic and environmental factors. As one of the malignant tumors with the highest mortality rates among women in the world, due to the heterogeneity and complexity of breast cancer, it is often difficult for a single treatment method to achieve the desired effect. Patients with early- and middle-stage breast cancer are mainly treated with surgery, and adjuvant treatment after surgery can reduce the risk of recurrence. Although the survival rate of patients with advanced breast cancer is very low, with the application of new targeted drugs, the survival period can be extended to 2–3 years [3]. Therefore, further study of clinical treatment methods for breast cancer is urgently needed.
In recent years, our understanding of mitochondria and mitochondrial dynamics has increased significantly; mitochondria not only provide energy to cells but also participate in important pathways, such as lipid metabolism, oxidative stress, cell differentiation, cell transmission, and apoptosis. An increasing number of studies have confirmed that mitochondria play an indelible role in cancer progression. Here, we describe the close relationship between mitochondrial dynamics and breast cancer progression, as well as the potential of targeting mitochondria in the treatment of breast cancer.
Mitochondria and mitochondrial dynamics
Mitochondria are double-membrane organelles that are involved mainly in oxidative phosphorylation (OXPHOS). The mitochondrial membrane comprises the outer mitochondrial membrane (OMM), the inner mitochondrial membrane (IMM), the mitochondrial membrane gap, and the mitochondrial matrix. It is the site of oxidative metabolism in eukaryotes and the main site of aerobic respiration in cells. It can synthesize ATP through oxidative phosphorylation [4] and can also promote intracellular Ca2+balance [5]. Mitochondria are also the main place where reactive oxygen species (ROS) are produced. It is mainly produced by complex i and complex II in the oxidative respiratory chain. ROS refers to the general term for oxygen-containing free radicals and peroxides that are easy to form free radicals related to oxygen metabolism in the organism. ROS can directly act on cytochrome C on the lipid membrane to release it, causing cell damage. ROS can also damage cell DNA and promote the formation of cancer cells (Fig. 1). In addition, mitochondria are involved in cell apoptosis and induce autophagy. In brief, mitochondria are essential for maintaining cellular homeostasis.
ROS is generally produced in complex I and complex II of the oxidized respiratory chain. It can release cytochrome C on the lipid membrane, causing cell damage. ROS can also directly damage cell DNA and promote tumor formation. a. IMM: Inner mitochondrial membrane; b. NADH: Nicotinamide adenine dinucleotide; c. FADH2: Flavine adenine dinucleotide; d. Cty-C: Cytochrome C; e. ROS: Reactive oxygen species
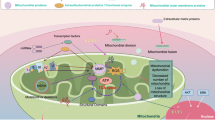
Mitochondrial dynamics refers to the dynamic balance between mitochondrial division and fusion. Most of the enzymes required are GTP enzymes, such as DRP1, MFN1/2, and OPA1, which are members of the Dynamin family [6] (Fig. 2). Mitochondrial dynamics are essential for maintaining the stability of mitochondrial function and not only affect the cell cycle during mitosis but also affect cell death [7, 8]. Studies have shown that mitochondrial dynamics play a key role in many diseases, such as degenerative diseases of the nervous system, cancer, diabetes, and cardiovascular ischaemia‒reperfusion injury [9].
Schematic diagram of mitochondrial dynamics. a. Drp 1: dynamin-related protein-1; b. Mfn 1: Mitofusin 1; c. Mfn 2: Mitofusin 2; d. Fis 1: Fission, Mitochondrial 1; e. Mff: mitochondrial fission factor; f. Mid49: mitochondrial dynamics proteins of 49 kDa; g. Mid51: mitochondrial dynamics proteins of 51 kDa; h. IMM: inner mitochondrial membrane
Mitochondrial fission refers to the process by which a mitochondrion divides into two mitochondria through multiple steps to adapt to cell survival. The motility protein required for mitochondrial fission is the GTP enzyme DRP1, which can be activated by the phosphorylation of the MAP kinases ERK1 and ERK2. Tong Xu et al. reported that when Porphyromonas gingivalis (Pg) infected endothelial cells and caused mitochondrial fragmentation, the phosphorylation of DRP1 at Ser616 significantly increased [10]. When mitochondria begin to undergo fission, DRP1 is recruited from the cytoplasm to the outer mitochondrial membrane and binds to the receptors Fis1, Mff, Mid49, and Mid51 to shrink the intima and outer membrane, thereby mediating the division of mitochondria [11,12,13]. In addition, Elena Smirnova reported that the inhibition of mitochondrial fission caused by DRP1 mutations can lead to the excessive fusion of tubular small bodies of mitochondria [14].
The order of mitochondrial fusion is generally outer membrane fusion first and then inner membrane fusion. During the fusion process, substances in mitochondria, such as mtDNA, proteins and metabolic substances, are exchanged. Three large GTP enzymes are needed: the outer membrane proteins MFN1 and MFN2 and the intimal protein OPA1 [15, 16]. MFN1 and MFN2 maintain their stability through the regulation of proteolytic ubiquitination [17]. The formation of the crest after mitochondrial fusion is usually related to the OPA1 protein [18]. Under normal circumstances, the fusion of the outer membrane and the inner membrane is balanced, but due to genetic mutations, the fusion of the inner membrane and the outer membrane can also occur independently of each other.
The role of mitochondrial dynamics in cancer
Mitochondrial dynamics may be related to cancer epithelial mesenchymal transformation, invasion, and metastasis [19]. An imbalance in mitochondrial dynamics, that is, an imbalance in fission and fusion, is an important factor leading to cancer progression [20,21,22,23]. In addition, Poorva Ghosh reported that increased mitochondrial fusion can promote oxidative phosphorylation and that increased fission can promote glycolysis [24].
Metastasis is an important cause of cancer death [25]. Compared with that of normal cells, the microenvironment of cancer cells generally presents a state of hypoxia in which there are many reactive oxygen species (ROS) [26]. Moreover, high levels of ROS promote mutations in mitochondrial DNA (mtDNA), changing mitochondrial function [27], and we also found that there are more fragmented mitochondria in metastatic cancer tissues; often, these cells have high DRP1 expression, while MFN is expressed at low levels. High DRP1 expression or low MFN expression results in mitochondrial fragmentation and enhances cancer metastasis [28]. In contrast, low DRP1 expression or high MFN expression causes mitochondria to become clustered or rod-shaped, thereby inhibiting the metastasis of cancer cells. Jalees Rehman discovered that inhibiting the fission of mitochondria prevents the metastasis of lung cancer cells (Table 1) [29].
Mitochondrial dynamics are closely related to the development of breast cancer
In recent years, increasing research has confirmed the important role of mitochondrial dynamics in the progression of breast cancer. By targeting Drp1 to inhibit mitochondrial fission, brain metastasis of breast cancer was alleviated [40]. In MDA-MB-231 cells treated with silymarin, the expression of MFN1/2 increased significantly, and the expression of DRP1 decreased, thereby promoting mitochondrial fusion and inhibiting the migration of breast cancer cells [41]. Estrogen is one of the important factors that cause breast cancer [42], Jorge Sastre-Serra et al. analyzed 17ß-oestradiol-treated MCF-7 cells by RT‒PCR and found that the expression of fusion mRNAs (Mfn1/2 and Opa1) increased and that of fission-related genes (Drp1 and Fis1) decreased, they unbalance mitochondrial fission and fusion, causing mitochondrial function to decrease [43]. IKK, an important member of the NF-kB pathway, has been extensively studied in recent years. It has been confirmed that it acts as a carcinogen and is related to the progression of a variety of cancers. Ruoyan Xu et al. reported that IKK2 inhibits the use of pyruvate in the tricarboxylic acid cycle in mitochondria in breast cancer cells, thereby causing mitochondrial dysfunction, inhibiting OXPHOS, and promoting the progression of breast cancer [44]. Phosphatidylserine decarboxylase (PISD) has been shown to promote mitochondrial fission [45]. With the exogenous addition of PISD to MDA-MB-231 cells, we found that the mitochondrial membrane potential changed and inhibited the growth of breast tumor cells (Table 2) [46].
Mitochondrial fission and breast cancer
Several studies have shown that by regulating the expression of mitochondrial fission factors, the proliferation and invasion of breast cancer cells can be suppressed to achieve therapeutic effects [48]. Moreover, highly expressed mitochondrial fission factors can also be used as prognostic indicators. By analyzing metastatic and invasive breast cancer cells, we found that the expression of DRP1 was greater in the former, which suggested that the upregulation of DRP1 expression may be an early signal of breast cancer metastasis [48].
One of the most critical steps in breast cancer progression before metastasis is the formation of lamellipodia at the leading edge of the cell [49]. The formation of these lamellipodia depends on actin and requires a large amount of ATP. The formation of lamellipodia at the leading edge of a cell refers to the formation of a sheet-like structure in the leading edge area to help cells move and invade tissue. The formation of this structure is essential for the metastasis of breast cancer cells because it can help them pass through the blood vessel wall or tissue gaps and enter blood vessels or lymphatic vessels, thereby facilitating metastasis to distant organs [50, 51] (Fig. 3). In in vitro experiments, we first used targeted siRNA transfection technology to reduce the expression of Drp1 in MDA-MB-231 breast cancer cells by 85%. Then we found that the diffusion rate of MDA-MB-231 cells with low or no expression of DRP1 protein was significantly reduced, and the percentage of plate-shaped pseudopods was reduced by about 60%. In contrast, cells with low Mfn1 protein expression exhibited accelerated diffusion and lamellipodium formation [48].
Mitochondrial fission regulator 1 (MTFR1) is a gene encoding a mitochondrial protein, and studies have shown that the abnormal expression of the MTFR1 protein is closely related to the occurrence and development of diseases such as tumors, neurodegenerative diseases, and metabolic diseases. By analyzing the tumor profile in a TCGA cohort, we found that MTFR1 is highly expressed in Her2-positive or ER-negative breast cancer and is associated with poor prognosis [52]. Moreover, we also found that high MTFR1 expression is negatively correlated with DNA methylation and that low MTFR1 methylation is often related to the occurrence of cancer [52]; however, the underlying mechanism is unclear. Guanming Lu also reported that mitochondrial fission regulator 2 (MTFR2) is dependent on HIF1a and HIF2a to transition from oxidative phosphorylation to glycolysis for glucose metabolism. An increase in the expression of this gene is also closely related to tumor progression in breast cancer cells [53].
Studies have proved that the Notch pathway plays an important role in regulating the metastasis of breast cancer stem cells. The expression of Notch1 mRNA is closely related to the poor prognosis of breast cancer [54], and there is a correlation between mitochondria and the Notch pathway. Li Chen et al. found that the activation of NICD1, the main functional region of Notch receptor, promotes the expression of the fission factor Drp1 in mitochondria, thereby promoting the metastasis of breast cancer cells [28] (Fig. 3). Therefore, γ-secretase inhibitors targeting the Notch pathway can be used for clinical treatment [55]. Several studies have shown that mitochondrial fission may affect the development of breast cancer by regulating pathways such as apoptosis and metabolic reprogramming [56]. Apoptosis is an important method of cell death that plays an important role in inhibiting the development of tumors. Abnormalities in mitochondrial fission may lead to abnormal signals in the apoptosis pathway [57], thereby promoting the growth and metastasis of breast cancer cells.
Mitochondrial fusion and breast cancer
The Warburg effect is regarded as an important feature of tumor cell energy metabolism [58]. Normal cells use oxygen to perform OXPHOS in the mitochondria to break down glucose to produce ATP, and cancer cells use glycolysis to breakdown glucose to produce lactic acid and ATP [59]. This effect is regarded as the Warburg effect. Tong Li et al. discovered that PKM2, the M2 subtype of pyruvate kinase, can bind to MFN2, thereby promoting mitochondrial fusion and oxidative phosphorylation and weakening the glycolytic process in cancer cells, thereby inhibiting the proliferation and metastasis of cancer cells [60]. mTOR is an atypical serine/threonine protein kinase and a member of the phosphatidylinositol kinase-related kinase (PIKK) protein family. By regulating the expression and activity of these fusion proteins, mTOR can affect the fusion and division of mitochondria. The mTOR-MFN2-PKM2 pathway mediated by mTOR enhances the interaction between MFN2 and PKM2, which is essential for glycolysis and oxidative phosphorylation in breast cancer cells (Fig. 3). However, high expression of the fusion protein Opa1 is related to poor prognosis in patients with breast cancer. Margherita Zamberlan reported that inhibiting Opa1 can reduce tumor growth, tumor aggressiveness, and neovascularization, thereby inhibiting the metastasis of breast cancer [61].
Mitochondria can be used as new targets for breast cancer treatment
For a long time, the emergence of targeted therapies for breast cancer has brought new hope to breast cancer patients. With the continuous deepening of research, targeted therapy drugs are also constantly being updated and improved (Table 3). Targeted mitochondrial therapy has been regarded as a focus of clinical research [24]. Mitochondrial fission is related to the poor prognosis of breast cancer patients. Therefore, directly or indirectly inhibiting the division of mitochondria inhibits the metastasis of breast cancer [62]. The mitochondrial division inhibitor Mdivi-1, which is a small molecule compound, can bind to DRP1 to inhibit mitochondrial fission, further reducing ATP production and oxidative phosphorylation, thereby promoting the apoptosis of breast cancer cells [63]. In addition, the peptide inhibitor P110 mainly prevents DRP1 from binding to the receptor Fis1, thereby inhibiting mitochondrial fission. Because of its fewer side effects, compared with Mdivi-1, P110 has better prospects in clinical applications.
Promoting mitochondrial fusion can improve the sensitivity of triple-negative breast cancer to chemotherapy. Studies have shown that in mice with MDA-MB231 breast cancer cells, the injection of P-Mito or Mdivi-1 can enhance sensitivity to doxorubicin to the same extent [47]. P-Mito is a polypeptide that can increase mitochondrial fusion and reduce oxidative stress. It has been shown to reduce cancer tissue metastasis and growth in TNBC mice [64].
The poor prognosis of triple-negative breast cancer is related to its high aggressiveness, and some studies have found that the ROS content in triple-negative breast cancer is higher than that in other types of breast cancer, and the strength of tumor aggressiveness is related to the content of ROS [65]. Therefore, eliminating the ROS content in cancer cells may attenuate the metastasis of cancer cells. Peroxide-reducing proteins (Prdxs) in mitochondria have been shown to be upregulated in different types of cancer, especially Prdxs 3 and Prdxs 5. But Prdxs 3 and Prdxs 5 may be associated with drug resistance in cancer. For example, the upregulation of Prdxs 3 in breast cancer is associated with azithromycin resistance [66], and the upregulation of Prdxs 5 in Hodgkin lymphoma is associated with resistance to azithromycin and vincristine [67]. Therefore, targeted therapy of Prdxs is currently a new research direction for cancer treatment, for example, curcumin, an inhibitor of Prdxs 6, has been shown to induce apoptosis of liver cancer cells to treat liver cancer [68]; Ainsliadimer A acts on Prdxs 1 and Prdxs 2 to treat colon cancer and many more [69].
Recent research has shown that mitochondrial transplantation may also become a new option for breast cancer treatment and may prevent a series of toxic side effects caused by traditional radiotherapy and chemotherapy. Jui-Chih Chang used cell-penetrating peptide (Pep-1) to mediate the transport of entire mitochondria to MCF-7 breast cancer cells. Without affecting the function of mitochondria, we found that this approach can reduce not only the biological activity of breast cancer cells [56] but also the occurrence of oxidative stress and drug resistance; however, the execution of this approach is complicated, and thus, this method has not been put into clinical use.
Perspectives and conclusion
In recent years, there has been increasing research on mitochondrial dynamics and cancer progression. In brief, the progression and metastasis of breast cancer are closely related to an imbalance in mitochondrial dynamics. Many experimental studies have shown that in metastatic breast cancer, mitochondrial fusion decreases, fission increases, and mitochondrial fragmentation increases. Further exploration of whether mitochondrial dynamics can truly treat breast cancer and provide targets is critical for clinical treatment. Future research should further clarify the mechanism of action of targeted mitochondrial therapy in the development of breast cancer and identify more effective treatment strategies to improve treatment efficacy in breast cancer patients.
Availability of data and materials
Data availability is not applicable to this article as no new data were created or analyzed in this study.
Abbreviations
- GTP:
-
Guanosine triphosphate
- Drp 1:
-
Dynamin-related protein-1
- Mfn 1:
-
Mitofusin 1
- Mfn 2:
-
Mitofusin 2
- Fis 1:
-
Fission, Mitochondrial 1
- Mff:
-
Mitochondrial fission factor
- Mid49:
-
Mitochondrial dynamics proteins of 49 kDa
- Mid51:
-
Mitochondrial dynamics proteins of 51 kDa
- RT‒PCR:
-
Reverse Transcription-Polymerase Chain Reaction
- PKM2:
-
Pyruvate kinase isozyme type M2
- TNBC:
-
Triple negative breast cancer
- ESCC:
-
Esophageal squamous cell carcinoma
- HNC:
-
Head and neck cancer
- HCC:
-
Hepatocellular carcinoma
- PDAC:
-
Pancreatic duct adenocarcinoma
- LUAD:
-
Lung adenocarcinoma
- FUNDC2:
-
FUN14 Domain Containing 2
- MST1:
-
Mammalian sterile 20-like kinase 1
- CSC:
-
Cancer stem cell
- IKKε:
-
IκB kinase ε
- Mdivi-1:
-
Mitochondrial division inhibitor 1
- PISD:
-
Phosphatidylserine decarboxylase
- NICD1:
-
Notch intracellular domain 1
- NADH:
-
Nicotinamide adenine dinucleotide
- FADH2:
-
Flavine adenine dinucleotide
- Cty-C:
-
Cytochrome C
- ROS:
-
Reactive oxygen species
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48.
Trayes KP, Cokenakes SEH. Breast cancer treatment. Am Fam Physician. 2021;104(2):171–8.
Leung AM, Vu HN, Nguyen KA, Thacker LR, Bear HD. Effects of surgical excision on survival of patients with stage IV breast cancer. J Surg Res. 2010;161(1):83–8.
von Jagow G, Engel WD. Structure and function of the energy-converting system of mitochondria. Angew Chem Int Ed Engl. 1980;19(9):659–75.
Szabadkai G, Duchen MR. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda). 2008;23:84–94.
van der Bliek AM, Shen Q, Kawajiri S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harb Perspect Biol. 2013;5(6).
Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11(12):872–84.
Maycotte P, Marin-Hernandez A, Goyri-Aguirre M, Anaya-Ruiz M, Reyes-Leyva J, Cortes-Hernandez P. Mitochondrial dynamics and cancer. Tumour Biol. 2017;39(5):1010428317698391.
Itoh K, Nakamura K, Iijima M, Sesaki H. Mitochondrial dynamics in neurodegeneration. Trends Cell Biol. 2013;23(2):64–71.
Xu T, Dong Q, Luo Y, Liu Y, Gao L, Pan Y, et al. Porphyromonas gingivalis infection promotes mitochondrial dysfunction through Drp1-dependent mitochondrial fission in endothelial cells. Int J Oral Sci. 2021;13(1):28.
Baeuerle PA, Murry JA. Human therapies as a successful liaison between chemistry and biology. Chem Biol. 2014;21(9):1046–54.
Kamerkar SC, Kraus F, Sharpe AJ, Pucadyil TJ, Ryan MT. Dynamin-related protein 1 has membrane constricting and severing abilities sufficient for mitochondrial and peroxisomal fission. Nat Commun. 2018;9(1):5239.
Kyriakoudi S, Drousiotou A, Petrou PP. When the balance tips: dysregulation of mitochondrial dynamics as a culprit in disease. Int J Mol Sci. 2021;22(9).
Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12(8):2245–56.
Miret-Casals L, Sebastian D, Brea J, Rico-Leo EM, Palacin M, Fernandez-Salguero PM, et al. Identification of new activators of mitochondrial fusion reveals a link between mitochondrial morphology and pyrimidine metabolism. Cell Chem Biol. 2018;25(3):268–78 e4.
Yang Z, Wang L, Yang C, Pu S, Guo Z, Wu Q, et al. Mitochondrial membrane remodeling. Front Bioeng Biotechnol. 2021;9: 786806.
Adaniya SM, O-Uchi J, Cypress MW, Kusakari Y, Jhun BS. Posttranslational modifications of mitochondrial fission and fusion proteins in cardiac physiology and pathophysiology. Am J Physiol Cell Physiol. 2019;316(5):C583–604.
Meeusen S, DeVay R, Block J, Cassidy-Stone A, Wayson S, McCaffery JM, et al. Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell. 2006;127(2):383–95.
Sharma A, Ahmad S, Ahmad T, Ali S, Syed MA. Mitochondrial dynamics and mitophagy in lung disorders. Life Sci. 2021;284: 119876.
Grandemange S, Herzig S, Martinou JC. Mitochondrial dynamics and cancer. Semin Cancer Biol. 2009;19(1):50–6.
Peiris-Pages M, Bonuccelli G, Sotgia F, Lisanti MP. Mitochondrial fission as a driver of stemness in tumor cells: mDIVI1 inhibits mitochondrial function, cell migration and cancer stem cell (CSC) signalling. Oncotarget. 2018;9(17):13254–75.
Chan DC. Mitochondrial dynamics and its involvement in disease. Annu Rev Pathol. 2020;15:235–59.
Lee YG, Park DH, Chae YC. Role of mitochondrial stress response in cancer progression. Cells. 2022;11(5).
Ghosh P, Vidal C, Dey S, Zhang L. Mitochondria targeting as an effective strategy for cancer therapy. Int J Mol Sci. 2020;21(9).
Eckhardt BL, Cao Y, Redfern AD, Chi LH, Burrows AD, Roslan S, et al. Activation of Canonical BMP4-SMAD7 Signaling Suppresses Breast Cancer Metastasis. Cancer Res. 2020;80(6):1304–15.
Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44(5):479–96.
Guha M, Avadhani NG. Mitochondrial retrograde signaling at the crossroads of tumor bioenergetics, genetics and epigenetics. Mitochondrion. 2013;13(6):577–91.
Chen L, Zhang J, Lyu Z, Chen Y, Ji X, Cao H, et al. Positive feedback loop between mitochondrial fission and Notch signaling promotes survivin-mediated survival of TNBC cells. Cell Death Dis. 2018;9(11):1050.
Rehman J, Zhang HJ, Toth PT, Zhang Y, Marsboom G, Hong Z, et al. Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. FASEB J. 2012;26(5):2175–86.
Li Y, Chen H, Yang Q, Wan L, Zhao J, Wu Y, et al. Increased Drp1 promotes autophagy and ESCC progression by mtDNA stress mediated cGAS-STING pathway. J Exp Clin Cancer Res. 2022;41(1):76.
Huang TL, Chang CR, Chien CY, Huang GK, Chen YF, Su LJ, et al. DRP1 contributes to head and neck cancer progression and induces glycolysis through modulated FOXM1/MMP12 axis. Mol Oncol. 2022;16(13):2585–606.
Liang J, Yang Y, Bai L, Li F, Li E. DRP1 upregulation promotes pancreatic cancer growth and metastasis through increased aerobic glycolysis. J Gastroenterol Hepatol. 2020;35(5):885–95.
Li S, Han S, Zhang Q, Zhu Y, Zhang H, Wang J, et al. FUNDC2 promotes liver tumorigenesis by inhibiting MFN1-mediated mitochondrial fusion. Nat Commun. 2022;13(1):3486.
Yeon SY, Jo YS, Choi EJ, Kim MS, Yoo NJ, Lee SH. Frameshift mutations in repeat sequences of ANK3, HACD4, TCP10L, TP53BP1, MFN1, LCMT2, RNMT, TRMT6, METTL8 and METTL16 genes in colon cancers. Pathol Oncol Res. 2018;24(3):617–22.
Hu Y, Wang B, Wang L, Wang Z, Jian Z, Deng L. Mammalian STE20-like kinase 1 regulates pancreatic cancer cell survival and migration through Mfn2-mediated mitophagy. Mol Med Rep. 2020;22(1):398–404.
Xu K, Chen G, Li X, Wu X, Chang Z, Xu J, et al. MFN2 suppresses cancer progression through inhibition of mTORC2/Akt signaling. Sci Rep. 2017;7:41718.
Pang G, Xie Q, Yao J. Mitofusin 2 inhibits bladder cancer cell proliferation and invasion via the Wnt/beta-catenin pathway. Oncol Lett. 2019;18(3):2434–42.
Carmona-Carmona CA, Dalla Pozza E, Ambrosini G, Cisterna B, Palmieri M, Decimo I, et al. Mitochondrial elongation and OPA1 play crucial roles during the stemness acquisition process in pancreatic ductal adenocarcinoma. Cancers (Basel). 2022;14(14).
Wang Y, Li Y, Jiang X, Gu Y, Zheng H, Wang X, et al. OPA1 supports mitochondrial dynamics and immune evasion to CD8(+) T cell in lung adenocarcinoma. PeerJ. 2022;10: e14543.
Parida PK, Marquez-Palencia M, Ghosh S, Khandelwal N, Kim K, Nair V, et al. Limiting mitochondrial plasticity by targeting DRP1 induces metabolic reprogramming and reduces breast cancer brain metastases. Nat Cancer. 2023;4(6):893–907.
Si L, Fu J, Liu W, Hayashi T, Nie Y, Mizuno K, et al. Silibinin inhibits migration and invasion of breast cancer MDA-MB-231 cells through induction of mitochondrial fusion. Mol Cell Biochem. 2020;463(1–2):189–201.
Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354(3):270–82.
Sastre-Serra J, Nadal-Serrano M, Pons DG, Roca P, Oliver J. Mitochondrial dynamics is affected by 17beta-estradiol in the MCF-7 breast cancer cell line. Effects on fusion and fission related genes. Int J Biochem Cell Biol. 2012;44(11):1901–5.
Xu R, Jones W, Wilcz-Villega E, Costa AS, Rajeeve V, Bentham RB, et al. The breast cancer oncogene IKKepsilon coordinates mitochondrial function and serine metabolism. EMBO Rep. 2020;21(9): e48260.
Chen YC, Humphries B, Brien R, Gibbons AE, Chen YT, Qyli T, et al. Functional isolation of tumor-initiating cells using microfluidic-based migration identifies phosphatidylserine decarboxylase as a key regulator. Sci Rep. 2018;8(1):244.
Humphries BA, Cutter AC, Buschhaus JM, Chen YC, Qyli T, Palagama DSW, et al. Enhanced mitochondrial fission suppresses signaling and metastasis in triple-negative breast cancer. Breast Cancer Res. 2020;22(1):60.
Chang JC, Chang HS, Yeh CY, Chang HJ, Cheng WL, Lin TT, et al. Regulation of mitochondrial fusion and mitophagy by intra-tumoral delivery of membrane-fused mitochondria or Midiv-1 enhances sensitivity to doxorubicin in triple-negative breast cancer. Biomed Pharmacother. 2022;153: 113484.
Zhao J, Zhang J, Yu M, Xie Y, Huang Y, Wolff DW, et al. Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene. 2013;32(40):4814–24.
Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773(5):642–52.
Xie Y, Wolff DW, Wei T, Wang B, Deng C, Kirui JK, et al. Breast cancer migration and invasion depend on proteasome degradation of regulator of G-protein signaling 4. Cancer Res. 2009;69(14):5743–51.
Kirui JK, Xie Y, Wolff DW, Jiang H, Abel PW, Tu Y. Gbetagamma signaling promotes breast cancer cell migration and invasion. J Pharmacol Exp Ther. 2010;333(2):393–403.
Fan WP, Du XN, Zhuang Y, Zhao XF, Sun HY, Zhang H. Expression and clinical significance of mitochondrial fission regulator 1 in breast invasive carcinoma analysed by high - throughput and multi -omics data. J Mod Oncol. 2019;27(12):2101–5. (in Chinece)
Lu G, Lai Y, Wang T, Lin W, Lu J, Ma Y, et al. Mitochondrial fission regulator 2 (MTFR2) promotes growth, migration, invasion and tumour progression in breast cancer cells. Aging (Albany NY). 2019;11(22):10203–19.
Reedijk M, Odorcic S, Chang L, Zhang H, Miller N, McCready DR, et al. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 2005;65(18):8530–7.
Song C, Zhang J, Xu C, Gao M, Li N, Geng Q. The critical role of gamma-secretase and its inhibitors in cancer and cancer therapeutics. Int J Biol Sci. 2023;19(16):5089–103.
Chang JC, Chang HS, Wu YC, Cheng WL, Lin TT, Chang HJ, et al. Mitochondrial transplantation regulates antitumour activity, chemoresistance and mitochondrial dynamics in breast cancer. J Exp Clin Cancer Res. 2019;38(1):30.
Yi L, Shang XJ, Lv L, Wang Y, Zhang J, Quan C, et al. Cadmium-induced apoptosis of Leydig cells is mediated by excessive mitochondrial fission and inhibition of mitophagy. Cell Death Dis. 2022;13(11):928.
Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269–70.
Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152.
Li T, Han J, Jia L, Hu X, Chen L, Wang Y. PKM2 coordinates glycolysis with mitochondrial fusion and oxidative phosphorylation. Protein Cell. 2019;10(8):583–94.
Zamberlan M, Boeckx A, Muller F, Vinelli F, Ek O, Vianello C, et al. Inhibition of the mitochondrial protein Opa1 curtails breast cancer growth. J Exp Clin Cancer Res. 2022;41(1):95.
Weiner-Gorzel K, Murphy M. Mitochondrial dynamics, a new therapeutic target for triple negative breast cancer. Biochim Biophys Acta Rev Cancer. 2021;1875(2): 188518.
Lucantoni F, Dussmann H, Prehn JHM. Metabolic Targeting of breast cancer cells with the 2-Deoxy-D-Glucose and the mitochondrial bioenergetics inhibitor MDIVI-1. Front Cell Dev Biol. 2018;6:113.
Chang JC, Chang HS, Wu YC, Cheng WL, Lin TT, Chang HJ, et al. Antitumor actions of intratumoral delivery of membrane-fused mitochondria in a mouse model of triple-negative breast cancers. Onco Targets Ther. 2020;13:5241–55.
Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68(6):1777–85.
Li L, Yu AQ. The functional role of peroxiredoxin 3 in reactive oxygen species, apoptosis, and chemoresistance of cancer cells. J Cancer Res Clin Oncol. 2015;141(12):2071–7.
Kropotov A, Gogvadze V, Shupliakov O, Tomilin N, Serikov VB, Tomilin NV, et al. Peroxiredoxin V is essential for protection against apoptosis in human lung carcinoma cells. Exp Cell Res. 2006;312(15):2806–15.
Chen J, Cao X, Qin X, Liu H, Chen S, Zhong S, et al. Proteomic analysis of the molecular mechanism of curcumin/beta-cyclodextrin polymer inclusion complex inhibiting HepG2 cells growth. J Food Biochem. 2020;44(2): e13119.
Lv C, Huang Y, Wang Q, Wang C, Hu H, Zhang H, et al. Ainsliadimer A induces ROS-mediated apoptosis in colorectal cancer cells via directly targeting peroxiredoxin 1 and 2. Cell Chem Biol. 2023;30(3):295–307 e5.
Liu A, Li Y, Lu S, Cai C, Zou F, Meng X. Stanniocalcin 1 promotes lung metastasis of breast cancer by enhancing EGFR-ERK-S100A4 signaling. Cell Death Dis. 2023;14(7):395.
Huang T, Meng F, Huang H, Wang L, Wang L, Liu Y, et al. GALNT8 suppresses breast cancer cell metastasis potential by regulating EGFR O-GalNAcylation. Biochem Biophys Res Commun. 2022;601:16–23.
Li ED, Lin Q, Meng YQ, Zhang LY, Song PP, Li N, et al. 2,4-Disubstituted quinazolines targeting breast cancer cells via EGFR-PI3K. Eur J Med Chem. 2019;172:36–47.
Manore SG, Doheny DL, Wong GL, Lo HW. IL-6/JAK/STAT3 Signaling in breast cancer metastasis: biology and treatment. Front Oncol. 2022;12: 866014.
Zhang J, Fan M, Jin C, Wang Z, Yao Y, Shi Y, et al. NFIC1 suppresses migration and invasion of breast cancer cells through interferon-mediated Jak-STAT pathway. Arch Biochem Biophys. 2022;727: 109346.
Hosford SR, Miller TW. Clinical potential of novel therapeutic targets in breast cancer: CDK4/6, Src, JAK/STAT, PARP, HDAC, and PI3K/AKT/mTOR pathways. Pharmgenomics Pers Med. 2014;7:203–15.
Ismail T, Kim Y, Lee H, Lee DS, Lee HS. Interplay between mitochondrial peroxiredoxins and ROS in cancer development and progression. Int J Mol Sci. 2019;20(18).
Jia H, Wang Z, Zhang J, Feng F. gamma-Secretase inhibitors for breast cancer and hepatocellular carcinoma: from mechanism to treatment. Life Sci. 2021;268: 119007.
Acknowledgements
Thanks for Dr. Pengcheng Xu for her guidance, inspiration or discussion on the topic selection, ideas, opinions, and arguments of the thesis, as well as for the revision opinions put forward during the review of the thesis.Thanks for Dr. Xu Pengcheng and Dr. Huiyi Tang for ttheir funding projects.
Funding
This work was supported by the Guangdong Province College Youth Innovative Talent Project [Grant No. 2022KQNCX037]; the National Natural Science Foundation of China [Grant No. 82102338].
Author information
Authors and Affiliations
Contributions
Jingwen Kuang: Writing—original draft, writing—review & editing. Hao Liu: Investigatio. Linlin Feng: Resources. Yuan Xue: Methodology, project administration. Huiyi Tang: Funding acquisition, supervision. Pengcheng Xu: Funding acquisition, supervision, validation, visualization.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
This article does not involve ethics.
Consent for publication
All authors of this article agree to the publication of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kuang, J., Liu, H., Feng, L. et al. How mitochondrial dynamics imbalance affects the progression of breast cancer:a mini review. Med Oncol 41, 238 (2024). https://doi.org/10.1007/s12032-024-02479-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-024-02479-2