Abstract
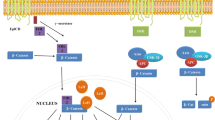
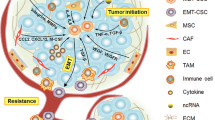
Despite recent advancements in the diagnosis and treatment of breast cancer (BC), patient outcomes in terms of survival, recurrence, and disease progression remain suboptimal. A significant factor contributing to these challenges is the cellular heterogeneity within BC, particularly the presence of breast cancer stem cells (BCSCs). These cells are thought to serve as the clonogenic nexus for new tumor growth, owing to their hierarchical organization within the tumor. This descriptive review focuses on the evolving strategies to target BCSCs, which have become a pivotal aspect of therapeutic development. We explore a variety of approaches, including targeting specific tumor surface markers (CD133 and CD44), transporters, heat shock proteins, and critical signaling pathways like Notch, Akt, Hedgehog, KLF4, and Wnt/β-catenin. Additionally, we discuss the modulation of the tumor microenvironment through the CXCR-12/CXCR4 axis, manipulation of pH levels, and targeting hypoxia-inducible factors, vascular endothelial growth factor, and CXCR1/2 receptors. Further, this review focuses on the roles of microRNA expression, strategies to induce apoptosis and differentiation in BCSCs, dietary interventions, dendritic cell vaccination, oncolytic viruses, nanotechnology, immunotherapy, and gene therapy. We particularly focused on studies reporting identification of BCSCs, their unique properties and the efficacy of various therapeutic modalities in targeting these cells. By dissecting these approaches, we aim to provide insights into the complex landscape of BC treatment and the potential pathways for improving patient outcomes through targeted BCSC therapies.




Similar content being viewed by others
Data availability
Not applicable for this review.
References
Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Organization, W.H. Preventing Cancer. 2020 [cited 2020 18-11-2020]; Available from: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2019.
Zhou J, et al. Stem cells and cellular origins of breast cancer: updates in the rationale, controversies, and therapeutic implications. Front Oncol. 2019;9:820–820.
Elbaiomy MA, et al. Clinical impact of breast cancer stem cells in metastatic breast cancer patients. J Oncol. 2020;2020:2561726.
Bianchini G, et al. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13(11):674–90.
Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306–13.
Tudoran OM, Balacescu O, Berindan-Neagoe I. Breast cancer stem-like cells: clinical implications and therapeutic strategies. Clujul Medical. 2016;89(2):193.
Dragu DL, et al. Therapies targeting cancer stem cells: current trends and future challenges. World J Stem Cells. 2015;7(9):1185.
Al-Hajj M, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–8.
Shipitsin M, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11(3):259–73.
Kai K, et al. Breast cancer stem cells. Breast Cancer. 2010;17(2):80–5.
Ma X, et al. Modulation of drug-resistant membrane and apoptosis proteins of breast cancer stem cells by targeting berberine liposomes. Biomaterials. 2013;34(18):4452–65.
Tam WL, et al. Protein kinase C α is a central signaling node and therapeutic target for breast cancer stem cells. Cancer Cell. 2013;24(3):347–64.
Singh JK, et al. Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res. 2013;15(4):210.
Gangopadhyay S, et al. Breast cancer stem cells: a novel therapeutic target. Clin Breast Cancer. 2013;13(1):7–15.
Glumac PM, LeBeau AM. The role of CD133 in cancer: a concise review. Clin Transl Med. 2018;7(1):18.
Vikram R, et al. Tumorigenic and metastatic role of CD44(-/low)/CD24(-/low) cells in luminal breast cancer. Cancers. 2020;12(5):1239.
Chen K, Huang Y-H, Chen J-L. Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol Sin. 2013;34(6):732.
Muntimadugu E, et al. CD44 targeted chemotherapy for co-eradication of breast cancer stem cells and cancer cells using polymeric nanoparticles of salinomycin and paclitaxel. Colloids Surf B Biointerfaces. 2016;143:532–46.
Daquan Chen GW, Song W, Zhang Q. Novel CD44 receptor targeting multifunctional “nano-eggs” based on double pH-sensitive nanoparticles for co-delivery of curcumin and paclitaxel to cancer cells and cancer stem cells. J Nanopart Res. 2015;17:10.
Najjar MK, Manore SG, Regua AT, Lo HW. Antibody-drug conjugates for the treatment of HER2-positive breast cancer. Genes (Basel). 2022;13:2065.
Luksik AS, Yazigi E, Shah P, Jackson CM. CAR T cell therapy in glioblastoma: overcoming challenges related to antigen expression. Cancers (Basel). 2023;15:1414.
Yanze Sun XY, Wang X, et al. Bispecific antibodies in cancer therapy: Target selection and regulatory requirements. Acta Pharm Sin B. 2023;13:3583–97.
Choi YH, Yu AM. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr Pharm Des. 2014;20(5):793–807.
Yue-Li Sun AP, Kumar P, Chen Z-S. Role of ABC transporters in cancer chemotherapy. Chin J Cancer. 2012;31(2):51.
Chuthapisith S, et al. Breast cancer chemoresistance: emerging importance of cancer stem cells. Surg Oncol. 2010;19(1):27–32.
Shivhare S, Das A. Cell density modulates chemoresistance in breast cancer cells through differential expression of ABC transporters. Mol Biol Rep. 2023;50(1):215–25.
Cui J, et al. Targeting ABCA12-controlled ceramide homeostasis inhibits breast cancer stem cell function and chemoresistance. Sci Adv. 2023;9(48):1891.
Wu CP, Ohnuma S, Ambudkar SV. Discovering natural product modulators to overcome multidrug resistance in cancer chemotherapy. Curr Pharm Biotechnol. 2011;12(4):609–20.
Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10(2):86–103.
Mao L. NOTCH mutations: multiple faces in human malignancies. Cancer Prev Res (Philadelphia, Pa). 2015;8:259–61.
Takebe N, Nguyen D, Yang SX. Targeting notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol Ther. 2014;141(2):140–9.
Aithal MG, Rajeswari N. Role of Notch signalling pathway in cancer and its association with DNA methylation. J Genet. 2013;92(3):667–75.
Harrison H, et al. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Can Res. 2010;70(2):709–18.
Hermawan A, et al. Bioinformatics and in vitro studies reveal the importance of p53, PPARG and notch signaling pathway in inhibition of breast cancer stem cells by hesperetin. Adv Pharm Bull. 2021;11(2):351.
Cejuela M, Martin-Castillo B, Menendez JA, Pernas S. Metformin and breast cancer: Where are we now? Int J Mol Sci. 2022;23(5):2705.
Rossini M, Martini F, Torreggiani E, Fortini F, Aquila G, Sega FV, Patergnani S, Pinton P, Maniscalco P, Cavallesco G, Rizzo P. Metformin induces apoptosis and inhibits notch1 in malignant pleural mesothelioma cells. Front Cell Dev Biol. 2021;8:534499.
Chia YH, Ma CX. Hedgehog pathway inhibitors: potential applications in breast cancer. Curr Breast Cancer Rep. 2011;3:15–23.
Tsao A-N, et al. Dinaciclib inhibits the stemness of two subtypes of human breast cancer cells by targeting the FoxM1 and Hedgehog signaling pathway. Oncol Rep. 2022;47(5):1–12.
Zhang Y, et al. Discovery of YH677 as a cancer stemness inhibitor that suppresses triple-negative breast cancer growth and metastasis by regulating the TGFbeta signaling pathway. Cancer Lett. 2023;560:216142.
Li Y, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 2010;16(9):2580–90.
Moynagh PN. The NF-κB pathway. J Cell Sci. 2005;118(20):4589–92.
Marquardt JU, et al. Curcumin effectively inhibits oncogenic NF-κB signaling and restrains stemness features in liver cancer. J Hepatol. 2015;63(3):661–9.
Zhou J, et al. NF-κB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res Treat. 2008;111(3):419–27.
Schreck R, et al. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992;175(5):1181–94.
MacKenzie CJ, et al. Enhancement of lipopolysaccharide-stimulated JNK activity in rat aortic smooth muscle cells by pharmacological and adenovirus-mediated inhibition of inhibitory kappa B kinase signalling. Br J Pharmacol. 2003;139(5):1041–9.
Clayton S, Mousa SA. Therapeutics formulated to target cancer stem cells: Is it in our future? Cancer Cell Int. 2011;11:7.
Gargini R, et al. Cancer stem cell-like phenotype and survival are coordinately regulated by Akt/FoxO/Bim pathway. Stem Cells. 2015;33(3):646–60.
Korkaya H, et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7(6):e1000121.
Ock CW, Kim GD. Dioscin decreases breast cancer stem-like cell proliferation via cell cycle arrest by modulating p38 mitogen-activated protein kinase and akt/mtor signaling pathways. J Cancer Prev. 2021;26(3):183.
Huang CC, et al. KLF4 and PCNA identify stages of tumor initiation in a conditional model of cutaneous squamous epithelial neoplasia. Cancer Biol Ther. 2005;4(12):1401–8.
Yu F, et al. Kruppel-like factor 4 (KLF4) is required for maintenance of breast cancer stem cells and for cell migration and invasion. Oncogene. 2011;30(18):2161.
Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–48.
Welschinger R, et al. Plerixafor (AMD3100) induces prolonged mobilization of acute lymphoblastic leukemia cells and increases the proportion of cycling cells in the blood in mice. Exp Hematol. 2012;41(3):293–302.
Prager GW, et al. Angiogenesis in cancer: Anti-VEGF escape mechanisms. Transl Lung Cancer Res. 2012;1(1):14–25.
Das B, et al. Quinacrine inhibits HIF-1α/VEGF-A mediated angiogenesis by disrupting the interaction between cMET and ABCG2 in patient-derived breast cancer stem cells. Phytomedicine. 2023;117:154914.
Zhao J, et al. The role of hypoxia-inducible factor-2 in digestive system cancers. Cell Death Dis. 2015;6(1):e1600.
Muthoni DK, et al. Identification of 3-O-α-l-arabinosyl oleanolic acid, a triterpenoid saponin, as a new breast cancer stem cell growth inhibitor. Nat Prod Res. 2022;36(11):2923–6.
Zhang C, et al. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m6A-demethylation of NANOG mRNA. Proc Natl Acad Sci U S A. 2016;113(14):E2047–56.
Chen M, et al. Extracellular pH is a biomarker enabling detection of breast cancer and liver cancer using CEST MRI. Oncotarget. 2017;8(28):45759–67.
Wojtkowiak JW, et al. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol Pharm. 2011;8(6):2032–8.
Chamie K, et al. Carbonic anhydrase-IX score is a novel biomarker that predicts recurrence and survival for high-risk, nonmetastatic renal cell carcinoma: data from the phase III ARISER clinical trial. Urol Oncol. 2015;33(5):204.e25-33.
Xu Z, et al. Preparation of a camptothecin prodrug with glutathione-responsive disulfide linker for anticancer drug delivery. Chem Asian J. 2014;9:199–205.
Lee ES, et al. Super pH-sensitive multifunctional polymeric micelle for tumor pH(e) specific TAT exposure and multidrug resistance. J Controll Release : Off J Controll Release Soc. 2008;129(3):228–36.
Ahuja SK, Murphy PM. The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J Biol Chem. 1996;271(34):20545–50.
Ginestier C, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120(2):485–97.
Singh JK, et al. Targeting CXCR1/2 significantly reduces breast cancer stem cell activity and increases the efficacy of inhibiting HER2 via HER2-dependent and -independent mechanisms. Clin Cancer Res. 2013;19(3):643–56.
Schott AF, et al. Phase Ib pilot study to evaluate reparixin in combination with weekly paclitaxel in patients with HER-2-negative metastatic breast cancer. Clin Cancer Res. 2017;23(18):5358–65.
Garg M. MicroRNAs, stem cells and cancer stem cells. World J Stem cells. 2012;4(7):62–70.
Yan LX, et al. Knockdown of miR-21 in human breast cancer cell lines inhibits proliferation, in vitro migration and in vivo tumor growth. Breast Cancer Res. 2011;13(1):R2.
Shimono Y, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138(3):592–603.
Iliopoulos D, et al. Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol Cell. 2010;39(5):761–72.
Gong C, et al. MiR-106b expression determines the proliferation paradox of TGF-β in breast cancer cells. Oncogene. 2015;34(1):84–93.
Juric et al., (2016) Ribociclib (LEE011) and letrozole in estrogen receptor-positive (ER+), HER2-negative (HERR ) advanced breast cancer (aBC): Phase Ib safety, preliminary efficacy and molecular analysis. 34, 568–568.
Cao T, et al. CDK3, target of miR-4469, suppresses breast cancer metastasis via inhibiting Wnt/β-catenin pathway. Oncotarget. 2017;8(49):84917–27.
Kong L-Y, et al. In vivo and in vitro effects of microRNA-27a on proliferation, migration and invasion of breast cancer cells through targeting of SFRP1 gene via Wnt/β-catenin signaling pathway. Oncotarget. 2017;8(9):15507–19.
Su X, et al. MicroRNA-134 targets KRAS to suppress breast cancer cell proliferation, migration and invasion. Oncol Lett. 2017;13(3):1932–8.
Yahya SM, et al. Restoring microRNA-34a overcomes acquired drug resistance and disease progression in human breast cancer cell lines via suppressing the ABCC1 gene. Breast Cancer Res Treat. 2023;204(1):133–49.
Zhao Y, et al. miR-29a suppresses MCF-7 cell growth by downregulating tumor necrosis factor receptor 1. Tumor Biol. 2017;39(2):1010428317692264.
Jasim SA, et al. An in vitro investigation of the apoptosis-inducing activity of corosolic acid in breast cancer cells. Iran J Basic Med Sci. 2023;26(4):453–60.
Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132(3):344–62.
Zhou J, et al. NF-kappaB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res Treat. 2008;111(3):419–27.
Gupta PB, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138(4):645–59.
Fuchs D, et al. Salinomycin induces apoptosis and overcomes apoptosis resistance in human cancer cells. Biochem Biophys Res Commun. 2009;390(3):743–9.
Ko D, et al. Anti-metastatic potential of pitavastatin in triple-negative breast cancer via targeting breast cancer stem-like properties and STAT3 signaling. Cancer Res. 2023;83(7):5800–5800.
Nalla LV, Khairnar A. Empagliflozin mediated miR-128-3p upregulation promotes differentiation of hypoxic cancer stem-like cells in breast cancer. Eur J Pharmacol. 2023;943:175565.
Yan Y, et al. All-trans retinoic acids induce differentiation and sensitize a radioresistant breast cancer cells to chemotherapy. BMC Complement Altern Med. 2016;16(1):113.
Jin X, Jin X, Kim H. Cancer stem cells and differentiation therapy. Tumor Biol. 2017;39(10):1010428317729933.
Yan Y, et al. All-trans retinoic acids induce differentiation and sensitize a radioresistant breast cancer cells to chemotherapy. BMC Complement Altern Med. 2016;16:113.
Patel SA, et al. Challenges in the development of future treatments for breast cancer stem cells. Breast cancer: Targ Ther. 2010;2:1.
Van Pham P, et al. Targeting breast cancer stem cells by dendritic cell vaccination in humanized mice with breast tumor: preliminary results. Onco Targets Ther. 2016;9:4441.
Shah D, Osipo C. Cancer stem cells and HER2 positive breast cancer: The story so far. Genes Dis. 2016;3(2):114–23.
Korkaya H, et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol Cell. 2012;47(4):570–84.
Montales MTE, et al. Repression of mammosphere formation of human breast cancer cells by soy isoflavone genistein and blueberry polyphenolic acids suggests diet-mediated targeting of cancer stem-like/progenitor cells. Carcinogenesis. 2012;33(3):652–60.
Higdon JV, et al. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55(3):224–36.
Ambrosone CB, et al. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr. 2004;134(5):1134–8.
Li Y, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res: Off J Am Assoc Cancer Res. 2010;16(9):2580–90.
Wang Y, et al. Differential effects of sulforaphane in regulation of angiogenesis in a co-culture model of endothelial cells and pericytes. Oncol Rep. 2017;37(5):2905–12.
Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269(2):291–304.
Zhang M, et al. Cancer stem cells as a potential therapeutic target in breast cancer. Stem Cell Investig. 2014. https://doi.org/10.3978/j.issn.2306-9759.2014.06.01.
Berzofsky JA, et al. Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J Clin Investig. 2004;113(11):1515–25.
Satthaporn S, et al. Dendritic cells are dysfunctional in patients with operable breast cancer. Cancer Immunol Immunother. 2004;53(6):510–8.
Ni J, et al. Dendritic cell vaccine for the effective immunotherapy of breast cancer. Biomed Pharmacother. 2020;126:110046.
Choi AH, et al. From benchtop to bedside: a review of oncolytic virotherapy. Biomedicines. 2016;4(3):18.
Russell SJ, Peng K-W, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30(7):658–70.
Rowan K. Oncolytic viruses move forward in clinical trials. JNCI J Natl Cancer Inst. 2010;102(9):590–5.
Marcato P, et al. Oncolytic reovirus effectively targets breast cancer stem cells. Mol Ther. 2009;17(6):972–9.
Wang H, et al. Oncolytic vaccinia virus GLV-1h68 strain shows enhanced replication in human breast cancer stem-like cells in comparison to breast cancer cells. J Transl Med. 2012;10:167.
Gomes EM, et al. Antitumor activity of an oncolytic adenoviral-CD40 ligand (CD154) transgene construct in human breast cancer cells. Clin Cancer Res. 2009;15(4):1317–25.
Zhang JF, et al. Treatment of a human breast cancer xenograft with an adenovirus vector containing an interferon gene results in rapid regression due to viral oncolysis and gene therapy. Proc Natl Acad Sci USA. 1996;93(9):4513–8.
Gil M, et al. Targeting CXCL12/CXCR4 signaling with oncolytic virotherapy disrupts tumor vasculature and inhibits breast cancer metastases. Proc Natl Acad Sci U S A. 2013;110(14):E1291–300.
He L, et al. Nanomedicine-mediated therapies to target breast cancer stem cells. Front Pharmacol. 2016;7:313–313.
Gao Y, et al. Nanotechnology-based intelligent drug design for cancer metastasis treatment. Biotechnol Adv. 2014;32(4):761–77.
Estanqueiro M, et al. Nanotechnological carriers for cancer chemotherapy: the state of the art. Colloids Surf B Biointerfaces. 2015;126:631–48.
Shen S, Xia JX, Wang J. Nanomedicine-mediated cancer stem cell therapy. Biomaterials. 2016;74:1–18.
Aires A, et al. Multifunctionalized iron oxide nanoparticles for selective drug delivery to CD44-positive cancer cells. Nanotechnology. 2016;27(6):065103.
Gener P, et al. Fluorescent CSC models evidence that targeted nanomedicines improve treatment sensitivity of breast and colon cancer stem cells. Nanomedicine. 2015;11(8):1883–92.
Ganesh S, et al. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials. 2013;34(13):3489–502.
Rao W, et al. Chitosan-decorated doxorubicin-encapsulated nanoparticle targets and eliminates tumor reinitiating cancer stem-like cells. ACS Nano. 2015;9(6):5725–40.
Gaio E, et al. CD44 targeting mediated by polymeric nanoparticles and combination of chlorine TPCS(2a)-PDT and docetaxel-chemotherapy for efficient killing of breast differentiated and stem cancer cells in vitro. Cancers. 2020;12(2):278.
Wang Z, et al. CD44 directed nanomicellar payload delivery platform for selective anticancer effect and tumor specific imaging of triple negative breast cancer. Nanomed Nanotechnol Biol Med. 2018;14(4):1441–54.
Zhang X, et al. Dual-responsive nanoparticles based on chitosan for enhanced breast cancer therapy. Carbohydr Polym. 2019;221:84–93.
Jeong K, et al. Development of highly efficient nanocarrier-mediated delivery approaches for cancer therapy. Cancer Lett. 2016;374(1):31–43.
Zhang L, et al. Mitochondrial targeting liposomes incorporating daunorubicin and quinacrine for treatment of relapsed breast cancer arising from cancer stem cells. Biomaterials. 2012;33(2):565–82.
Yu LY, et al. Glucose transporter 1-mediated transcytosis of glucosamine-labeled liposomal ceramide targets hypoxia niches and cancer stem cells to enhance therapeutic efficacy. ACS Nano. 2023;17(14):13158–75.
Nguyen VD, et al. Folate receptor-targeted liposomal nanocomplex for effective synergistic photothermal-chemotherapy of breast cancer in vivo. Colloids Surf B Biointerfaces. 2019;173:539–48.
Chiani M, et al. Folic acid conjugated nanoliposomes as promising carriers for targeted delivery of bleomycin. Artif Cells Nanomed Biotechnol. 2018;46(4):757–63.
Kushwah V, et al. Improved antitumor efficacy and reduced toxicity of docetaxel using anacardic acid functionalized stealth liposomes. Colloids Surf B Biointerfaces. 2018;172:213–23.
Hong S, et al. Aptamer-integrated α-Gal liposomes as bispecific agents to trigger immune response for killing tumor cells. J Biomed Mater Res A. 2019;107(6):1176–83.
Kim YJ, et al. Co-eradication of breast cancer cells and cancer stem cells by cross-linked multilamellar liposomes enhances tumor treatment. Mol Pharm. 2015;12(8):2811–22.
Rana MS, et al. A new liposomal nanocarrier for co-delivery of gedunin and p-glycoprotein siRNA to target breast cancer stem cells. Nat Prod Res. 2022;36(24):6389–92.
Ahmad A, et al. Development of liposomal formulation for delivering anticancer drug to breast cancer stem-cell-like cells and its pharmacokinetics in an animal model. Mol Pharm. 2016;13(3):1081–8.
Zhang Y, et al. The eradication of breast cancer and cancer stem cells using octreotide modified paclitaxel active targeting micelles and salinomycin passive targeting micelles. Biomaterials. 2012;33(2):679–91.
Ke XY, et al. Co-delivery of thioridazine and doxorubicin using polymeric micelles for targeting both cancer cells and cancer stem cells. Biomaterials. 2014;35(3):1096–108.
Zhang J, et al. Effective treatment of drug resistant recurrent breast tumors harboring cancer stem-like cells by staurosporine/epirubicin co-loaded polymeric micelles. J Control Release. 2017;264:127–35.
Xiang J, et al. Synthesis and evaluation of a paclitaxel-binding polymeric micelle for efficient breast cancer therapy. Sci China Life Sci. 2018;61(4):436–47.
Lecot et al., Glucosylated polymeric micelles actively target a breast cancer model. n/a(n/a), 2000010
Amreddy N, et al. Recent advances in nanoparticle-based cancer drug and gene delivery. Adv Cancer Res. 2018;137:115–70.
Sun R, et al. Co-delivery of all-trans-retinoic acid and doxorubicin for cancer therapy with synergistic inhibition of cancer stem cells. Biomaterials. 2015;37:405–14.
de Sousa Marcial SP, Carneiro G, Leite EA. Lipid-based nanoparticles as drug delivery system for paclitaxel in breast cancer treatment. J Nanoparticle Res. 2017;19(10):340.
Wong MY, Chiu GN. Liposome formulation of co-encapsulated vincristine and quercetin enhanced antitumor activity in a trastuzumab-insensitive breast tumor xenograft model. Nanomedicine. 2011;7(6):834–40.
Şalva E, et al. The enhancement of gene silencing efficiency with chitosan-coated liposome formulations of siRNAs targeting HIF-1α and VEGF. Int J Pharm. 2015;478(1):147–54.
Li SY, et al. Combination therapy with epigenetic-targeted and chemotherapeutic drugs delivered by nanoparticles to enhance the chemotherapy response and overcome resistance by breast cancer stem cells. J Control Release. 2015;205:7–14.
Jin G, et al. Theranostics of triple-negative breast cancer based on conjugated polymer nanoparticles. ACS Appl Mater Interfaces. 2018;10(13):10634–46.
Shen S, et al. Delivery of bortezomib with nanoparticles for basal-like triple-negative breast cancer therapy. J Control Release. 2015;208:14–24.
Sun R, et al. Nanoparticle-facilitated autophagy inhibition promotes the efficacy of chemotherapeutics against breast cancer stem cells. Biomaterials. 2016;103:44–55.
Gao J, et al. Co-delivery of docetaxel and salinomycin to target both breast cancer cells and stem cells by PLGA/TPGS nanoparticles. Int J Nanomed. 2019;14:9199–216.
Jeon M, et al. Paclitaxel-loaded iron oxide nanoparticles for targeted breast cancer therapy. Adv Ther. 2019;2(12):1900081.
Tran T, et al. Synergistic therapeutic strategy of dual drug-loaded lipid polymer hybrid nanoparticles for breast cancer treatment. Indian J Pharm Sci. 2019;81:474–82.
Esnaashari SS, et al. A combinational approach towards treatment of breast cancer: an analysis of noscapine-loaded polymeric nanoparticles and doxorubicin. AAPS PharmSciTech. 2020;21(5):166.
Sulaiman A, et al. Co-targeting bulk tumor and CSCs in clinically translatable tnbc patient-derived xenografts via combination nanotherapy. Mol Cancer Ther. 2019;18(10):1755.
Zhang N, et al. Loading lovastatin into camptothecin-floxuridine conjugate nanocapsules for enhancing anti-metastatic efficacy of cocktail chemotherapy on triple-negative breast cancer. ACS Appl Mater Interfaces. 2018;10(35):29385–97.
Liang DS, et al. Vitamin E-based redox-sensitive salinomycin prodrug-nanosystem with paclitaxel loaded for cancer targeted and combined chemotherapy. Colloids Surf B Biointerfaces. 2018;172:506–16.
Tran TA, et al. Inhibitory effect of zinc sulfide nanoparticles towards breast cancer stem cell migration and invasion. J Biomed Nanotechnol. 2016;12(2):329–36.
Dash SR, et al. Near-infrared enhances antiangiogenic potentiality of quinacrine-gold hybrid nanoparticles in breast cancer stem cells via deregulation of HSP-70/TGF-beta. Nanomedicine (Lond). 2023;18(1):19–33.
Wu K, et al. An iron oxyhydroxide-based nanosystem sensitizes ferroptosis by a “Three-Pronged” strategy in breast cancer stem cells. Acta Biomater. 2023;160:281–96.
Sarika PR, Nirmala RJ. Curcumin loaded gum arabic aldehyde-gelatin nanogels for breast cancer therapy. Mater Sci Eng, C. 2016;65:331–7.
Guo H, et al. Chitosan-based nanogel enhances chemotherapeutic efficacy of 10-hydroxycamptothecin against human breast cancer cells. Int J Polym Sci. 2019;2019:1914976.
Gülçür E, et al. Curcumin in VIP-targeted sterically stabilized phospholipid nanomicelles: a novel therapeutic approach for breast cancer and breast cancer stem cells. Drug Deliv Transl Res. 2013;3(6):562–74. https://doi.org/10.1007/s13346-013-0167-6.
Dian L-H, et al. Fabrication of paclitaxel hybrid nanomicelles to treat resistant breast cancer via oral administration. Int J Nanomed. 2018;13:719–31.
Gong Z, et al. Fibronectin-targeted dual-acting micelles for combination therapy of metastatic breast cancer. Signal Transduct Target Ther. 2020;5(1):12.
Farhadi S, et al. Exosomal delivery of 7SK long non-coding RNA suppresses viability, proliferation, aggressiveness and tumorigenicity in triple negative breast cancer cells. Life Sci. 2023;322:121646.
Ohno S-I, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21(1):185–91.
Yang M, et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10(1):117.
Mina LA, et al. Immunotherapy for the treatment of breast cancer: emerging new data. Breast cancer (Dove Medical Press). 2019;11:321–8.
Yang M, et al. Mesothelin-targeted CAR-NK cells derived from induced pluripotent stem cells have a high efficacy in killing triple-negative breast cancer cells as shown in several preclinical models. J Immunother. 2023;46(8):285–94.
Nanda R, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34(21):2460–7.
Dirix LY, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN solid tumor study. Breast Cancer Res Treat. 2018;167(3):671–86.
Schmid P, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–21.
Adams S, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):397–404.
Schmid P, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med. 2020;382(9):810–21.
Lang J-Y, et al. BikDD eliminates breast cancer initiating cells and synergizes with lapatinib for breast cancer treatment. Cancer Cell. 2011;20(3):341–56.
Thapa B, et al. Breathing new life into TRAIL for breast cancer therapy: co-delivery of pTRAIL and complementary siRNAs using lipopolymers. Hum Gene Ther. 2019;30(12):1531–46.
Kretzmann JA, et al. Tumor suppression by targeted intravenous non-viral CRISPRa using dendritic polymers. Chem Sci. 2019;10(33):7718–27.
Moradian C, Rahbarizadeh F. Targeted toxin gene therapy of breast cancer stem cells using CXCR1 Promoter and bFGF 5’UTR. Onco Targets Ther. 2019;12:8809–20.
Zuo ZQ, et al. Promoting tumor penetration of nanoparticles for cancer stem cell therapy by TGF-beta signaling pathway inhibition. Biomaterials. 2016;82:48–59.
Agency FD. New Drug Approvals for 2019. 2019 [cited 2020 18-11-2020]; Available from: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2019.
Agency FD, Novel drug approvals for 2020. 2020 [cited 2020 18-11-2020]; Available from: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2020.
Acknowledgements
None
Funding
This work received no funding.
Author information
Authors and Affiliations
Contributions
First two authors contributed equally to this work. KA and MN wrote the manuscript and conceived the idea, edited the manuscript and worked on nano-technology approaches, gene therapy and immunotherapy and diet mediate therapy. FM provided in-depth information about the signaling pathways and made that figure, and edited the manuscript; MIQ and MI contributed in providing valuable information on surface receptors and transporters, revised the manuscript, and tabulated the studies on nanocarriers; MFR and FKH edited the manuscript and wrote tumor microenvironment and miRNA-based approaches; SAH prepared figures for the manuscript and edited the manuscript; HS supervised the work, edited the manuscript and tabulated the results on surface receptor targeting, and provided literature searches. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ali, K., Nabeel, M., Mohsin, F. et al. Recent developments in targeting breast cancer stem cells (BCSCs): a descriptive review of therapeutic strategies and emerging therapies. Med Oncol 41, 112 (2024). https://doi.org/10.1007/s12032-024-02347-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-024-02347-z