Abstract
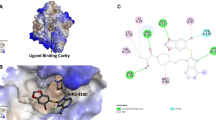
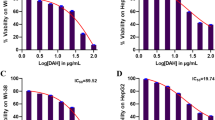
Combination therapy has been frequently preferred in treating various types of cancer in recent years. Targeted cancer therapy has also become one of the remarkable treatment modalities. Therefore, the aim of the study to investigate its cytotoxic and apoptotic effects on liver cancer cell lines by combining the classical chemotherapeutic drug doxorubicin (DOX) and a targeted agent, the new generation HSP90 inhibitor XL-888. The molecular docking method was used to predict the binding conformation of XL-888 on the human Hsp90. The single and combined cytotoxic effects of DOX and XL-888 on liver cancer cell lines HepG2 and HUH-7 were determined by MTT assay. The effect of the combined use of two drugs was evaluated using Chou and Talalay method. The levels of apoptotic genes and heat shock proteins gene and protein expression levels were investigated by quantitative real-time polymerase chain reaction and western blotting, respectively. Molecular docking results showed that XL-888 selectively binds to the ATP binding pocket of HSP90 with an estimated free binding energy of − 7.8 kcal/mol. DOX and XL-888 and their combination showed dose-dependent cytotoxic effect. The combination of drugs showed a synergistic effect on both cell lines. The results revealed that the combination of DOX and XL-888 potently induced apoptosis in liver cancer cell lines rather than using drugs alone. The combined treatment of DOX and XL-888 demonstrated synergistic cytotoxic and apoptotic effects on liver cancer cell lines, presenting a promising approach for combination therapy in liver cancer.





Similar content being viewed by others
Data availability
Data and materials are available from the author upon request.
References
Hajiasgharzadeh K, Somi MH, Shanehbandi D, Mokhtarzadeh A, Baradaran B. Small interfering RNA-mediated gene suppression as a therapeutic intervention in hepatocellular carcinoma. J Cell Physiol. 2019;234:3263–76. https://doi.org/10.1002/jcp.27015.
Chen S, Cao Q, Wen W, Wang H. Targeted therapy for hepatocellular carcinoma: challenges and opportunities. Cancer Lett. 2019;460:1–9. https://doi.org/10.1016/j.canlet.2019.114428.
Ferenci P, Fried M, Labrecque D, Bruix J, Sherman M, Omata M, et al. Hepatocellular carcinoma (HCC): a global perspective. J Clin Gastroenterol. 2010;44:239–45. https://doi.org/10.1097/MCG.0b013e3181d46ef2.
Le Grazie M, Biagini MR, Tarocchi M, Polvani S, Galli A. Chemotherapy for hepatocellular carcinoma: the present and the future. World J Hepatol. 2017;9:907–20. https://doi.org/10.4254/wjh.v9.i21.907.
Cagel M, Grotz E, Bernabeu E, Moretton MA, Chiappetta DA. Doxorubicin: nanotechnological overviews from bench to bedside. Drug Discovery Today. 2017;22:270–81. https://doi.org/10.1016/j.drudis.2016.11.005.
Radu ER, Semenescu A, Voicu SI. Recent advances in stimuli-responsive doxorubicin delivery systems for liver cancer therapy. Polymers. 2022;14:5249.
Mohammadi M, Arabi L, Alibolandi M. Doxorubicin-loaded composite nanogels for cancer treatment. J Control Release. 2020;328:171–91. https://doi.org/10.1016/j.jconrel.2020.08.033.
Yue W, Yupeng G, Jun C, Kui J. Apatinib combined with olaparib induces ferroptosis via a p53-dependent manner in ovarian cancer. J Cancer Res Clin Oncol. 2023;149:8681–9. https://doi.org/10.1007/s00432-023-04811-1.
Sturm M-J, Henao-Restrepo JA, Becker S, Proquitté H, Beck JF, Sonnemann J. Synergistic anticancer activity of combined ATR and ribonucleotide reductase inhibition in Ewing’s sarcoma cells. J Cancer Res Clin Oncol. 2023;149:8605–17. https://doi.org/10.1007/s00432-023-04804-0.
Özgür A, Tutar Y. Heat shock protein 90 inhibition in cancer drug discovery: from chemistry to futural clinical applications. Anticancer Agents Med Chem. 2016;16:280–90. https://doi.org/10.2174/1871520615666150821093747.
Verma S, Goyal S, Jamal S, Singh A, Grover A. Hsp90: friends, clients and natural foes. Biochimie. 2016;127:227–40. https://doi.org/10.1016/j.biochi.2016.05.018.
Ren X, Li T, Zhang W, Yang X. Targeting heat-shock protein 90 in cancer: an update on combination therapy. Cells. 2022;11:2556.
Qin L, Huang H, Huang J, Wang G, Huang J, Wu X, et al. Biological characteristics of heat shock protein 90 in human liver cancer cells. Am J Transl Res. 2019;11:2477–83.
Wei W, Liu M, Ning S, Wei J, Zhong J, Li J, et al. Diagnostic value of plasma HSP90α levels for detection of hepatocellular carcinoma. BMC Cancer. 2020;20:6. https://doi.org/10.1186/s12885-019-6489-0.
Özgür A. Investigation of anticancer activities of STA-9090 (ganetespib) as a second generation HSP90 inhibitor in Saos-2 osteosarcoma cells. J Chemother. 2021;33:554–63. https://doi.org/10.1080/1120009X.2021.1908650.
Ozgur A, Tutar Y. Heat shock protein 90 inhibitors in oncology. Curr Proteomics. 2014;11:2–16.
Bussenius J, Blazey CM, Aay N, Anand NK, Arcalas A, Baik T, et al. Discovery of XL888: a novel tropane-derived small molecule inhibitor of HSP90. Bioorg Med Chem Lett. 2012;22:5396–404. https://doi.org/10.1016/j.bmcl.2012.07.052.
Zhang Y, Ware MB, Zaidi MY, Ruggieri AN, Olson BM, Komar H, et al. Heat shock protein-90 inhibition alters activation of pancreatic stellate cells and enhances the efficacy of PD-1 blockade in pancreatic cancer. Mol Cancer Ther. 2021;20:150–60. https://doi.org/10.1158/1535-7163.Mct-19-0911.
Vido MJ, Aplin AE. The broad stroke of Hsp90 inhibitors: painting over the RAF inhibitor paradox. J Investig Dermatol. 2015;135:2355–7. https://doi.org/10.1038/jid.2015.239.
Tosun NG, Kaplan Ö, Özgür A. Apoptosis induced by Tarantula cubensis crude venom (Theranekron® D6) in cancer cells. Rev Bras. 2021;31:824–31. https://doi.org/10.1007/s43450-021-00221-x.
Kaplan Ö, Gökşen Tosun N, İmamoğlu R, Türkekul İ, Gökçe İ, Özgür A. Biosynthesis and characterization of silver nanoparticles from Tricholoma ustale and Agaricus arvensis extracts and investigation of their antimicrobial, cytotoxic, and apoptotic potentials. J Drug Deliv Sci Technol. 2022;69:103178. https://doi.org/10.1016/j.jddst.2022.103178.
Schicht G, Seidemann L, Haensel R, Seehofer D, Damm G. Critical investigation of the usability of hepatoma cell lines HepG2 and Huh7 as models for the metabolic representation of resectable hepatocellular carcinoma. Cancers (Basel). 2022. https://doi.org/10.3390/cancers14174227.
Chou T-C. Frequently asked questions in drug combinations and the mass-action law-based answers. Synergy. 2014;1:3–21. https://doi.org/10.1016/j.synres.2014.07.003.
Chou T-C. The combination index (CI < 1) as the definition of synergism and of synergy claims. Synergy. 2018;7:49–50. https://doi.org/10.1016/j.synres.2018.04.001.
Muller P, Hrstka R, Coomber D, Lane DP, Vojtesek B. Chaperone-dependent stabilization and degradation of p53 mutants. Oncogene. 2008;27:3371–83. https://doi.org/10.1038/sj.onc.1211010.
Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–49. https://doi.org/10.1038/nrc2887.
Augello G, Emma MR, Cusimano A, Azzolina A, Mongiovì S, Puleio R, et al. Targeting HSP90 with the small molecule inhibitor AUY922 (luminespib) as a treatment strategy against hepatocellular carcinoma. Int J Cancer. 2019;144:2613–24. https://doi.org/10.1002/ijc.31963.
Wang C, Zhang Y, Guo K, Wang N, Jin H, Liu Y, et al. Heat shock proteins in hepatocellular carcinoma: molecular mechanism and therapeutic potential. Int J Cancer. 2016;138:1824–34. https://doi.org/10.1002/ijc.29723.
Ramalingam M, Jang S, Jeong HS. Therapeutic effects of conditioned medium of neural differentiated human bone marrow-derived stem cells on rotenone-induced alpha-synuclein aggregation and apoptosis. Stem Cells Int. 2021;2021:6658271. https://doi.org/10.1155/2021/6658271.
Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873:188314. https://doi.org/10.1016/j.bbcan.2019.188314.
Daunys S, Matulis D, Petrikaitė V. Synergistic activity of Hsp90 inhibitors and anticancer agents in pancreatic cancer cell cultures. Sci Rep. 2019;9:16177. https://doi.org/10.1038/s41598-019-52652-1.
Gupta B, Pathak S, Poudel BK, Regmi S, Ruttala HB, Gautam M, et al. Folate receptor-targeted hybrid lipid-core nanocapsules for sequential delivery of doxorubicin and tanespimycin. Colloids Surf B. 2017;155:83–92. https://doi.org/10.1016/j.colsurfb.2017.04.010.
Lai CH, Park KS, Lee DH, Alberobello AT, Raffeld M, Pierobon M, et al. HSP-90 inhibitor ganetespib is synergistic with doxorubicin in small cell lung cancer. Oncogene. 2014;33:4867–76. https://doi.org/10.1038/onc.2013.439.
Akce M, Alese OB, Shaib WL, Wu C, Lesinski GB, El-Rayes BF. Phase Ib trial of pembrolizumab and XL888 in patients with advanced gastrointestinal malignancies: results of the dose-escalation phase. J Clin Oncol. 2020;38:830–830. https://doi.org/10.1200/JCO.2020.38.4_suppl.830.
Eroglu Z, Chen YA, Gibney GT, Weber JS, Kudchadkar RR, Khushalani NI, et al. Combined BRAF and HSP90 inhibition in patients with unresectable BRAF V600E-mutant melanoma. Clin Cancer Res. 2018;24:5516–24.
Mooradian M, Cleary JM, Cohen JV, Lawrence DP, Buchbinder EI, Giobbie-Hurder A, et al. CTEP 9557: a dose-escalation trial of combination dabrafenib, trametinib, and AT13387 in patients with BRAF mutant solid tumors. J Clin Oncol. 2020;38(15_Suppl):3609.
Sun C, Bai M, Ke W, Wang X, Zhao X, Lu Z. The HSP90 inhibitor, XL888, enhanced cell apoptosis via downregulating STAT3 after insufficient radiofrequency ablation in hepatocellular carcinoma. Life Sci. 2021;282:119762. https://doi.org/10.1016/j.lfs.2021.119762.
Acknowledgements
The author would like to thank Prof. Dr. İsa Gökçe and Dr. Nazan Gökşen Tosun for contributions.
Funding
No support was received in this study. However, the facilities of Tokat Gaziosmanpasa University, Faculty of Engineering and Architecture, Department of Bioengineering laboratories were used.
Author information
Authors and Affiliations
Contributions
ÖK: Investigation, methodology, formal analysis, writing—review and editing, visualization, methodology, resources.
Corresponding author
Ethics declarations
Conflict of interest
The author confirm that this article content has no conflicts of interest.
Ethical approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaplan, Ö. Synergistic induction of apoptosis in liver cancer cells: exploring the combined potential of doxorubicin and XL-888. Med Oncol 40, 318 (2023). https://doi.org/10.1007/s12032-023-02181-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02181-9