Abstract
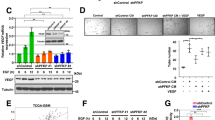
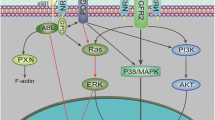
Platelet-derived growth factor receptor-β (PDGFRβ) is a critical type III receptor tyrosine kinase family member, which is involved in Wilms’ tumour (WT) metastasis and aerobic glycolysis. The role of PDGFRβ in tumour angiogenesis has not been fully elucidated. Here, we examined the effect of PDGFRβ on angiogenesis in WT. First, the NCBI database integrated three datasets, GSE2712, GSE11151, and GSE73209, to screen differentially expressed genes. The R language was used to analyse the correlation between PDGFRB and vascular endothelial growth factor (VEGF). The results showed that PDGFRB, encoding PDGFRβ, was upregulated in WT, and its level was correlated with VEGFA expression. Next, PDGFRβ expression was inhibited by small interfering RNA (siRNA) or activated with the exogenous ligand PDGF-BB. The expression and secretion of the angiogenesis elated factor VEGFA in WT G401 cells were detected using Western blotting and ELISA, respectively. The effects of conditioned medium from G401 cells on endothelial cell viability, migration, invasion, the total length of the tube, and the number of fulcrums were investigated. To further explore the mechanism of PDGFRβ in the angiogenesis of WT, the expression of VEGFA was detected after blocking the phosphatidylinositol-3-kinase (PI3K) pathway and inhibiting the expression of PKM2, a key enzyme of glycolysis. The results indicated that PDGFRβ regulated the process of tumour angiogenesis through the PI3K/AKT/PKM2 pathway. Therefore, this study provides a novel therapeutic strategy to target PDGFRβ and PKM2 to inhibit glycolysis and anti-angiogenesis, thus, developing a new anti-vascular therapy.






Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Nakata K, Colombet M, Stiller CA, Pritchard-Jones K, Steliarova-Foucher E. Incidence of childhood renal tumours: an international population-based study. Int J Cancer. 2020;147(12):3313–27.
Spreafico F, Fernandez CV, Brok J, Nakata K, Vujanic G, Geller JI, Gessler M, Maschietto M, Behjati S, Polanco A, Paintsil V, Luna-Fineman S, Pritchard-Jones K. Wilms tumour. Nat Rev Dis Primers. 2021;7(1):75.
Brok J, Treger TD, Gooskens SL, van den Heuvel-Eibrink MM, Pritchard-Jones K. Biology and treatment of renal tumours in childhood. Eur J Cancer (Oxford). 2016;68:179–95.
Dome JS, Mullen EA, Dix DB, Gratias EJ, Ehrlich PF, Daw NC, Geller JI, Chintagumpala M, Khanna G, Kalapurakal JA, Renfro LA, Perlman EJ, Grundy PE, Fernandez CV. Impact of the first generation of Children’s Oncology Group Clinical trials on clinical practice for Wilms tumor. J Natl Compr Cancer Netw. 2021;19(8):978–85.
Mullen EA, Chi Y-Y, Hibbitts E, Anderson JR, Steacy KJ, Geller JI, Green DM, Khanna G, Malogolowkin MH, Grundy PE, Fernandez CV, Dome JS. Impact of surveillance imaging modality on survival after recurrence in patients with favorable-histology wilms tumor: a report from the Children’s Oncology Group. J Clin Oncol. 2018;36:3396.
Suh E, Stratton KL, Leisenring WM, Nathan PC, Ford JS, Freyer DR, McNeer JL, Stock W, Stovall M, Krull KR, Sklar CA, Neglia JP, Armstrong GT, Oeffinger KC, Robison LL, Henderson TO. Late mortality and chronic health conditions in long-term survivors of early-adolescent and young adult cancers: a retrospective cohort analysis from the Childhood Cancer Survivor Study. Lancet Oncol. 2020;21(3):421–35.
van den Heuvel-Eibrink MM, Hol JA, Pritchard-Jones K, van Tinteren H, Furtwängler R, Verschuur AC, Vujanic GM, Leuschner I, Brok J, Rübe C, Smets AM, Janssens GO, Godzinski J, Ramírez-Villar GL, de Camargo B, Segers H, Collini P, Gessler M, Bergeron C, Spreafico F, Graf N. Position paper: rationale for the treatment of Wilms tumour in the UMBRELLA SIOP-RTSG 2016 protocol. Nat Rev Urol. 2017;14(12):743–52.
Majidpoor J, Mortezaee K. Angiogenesis as a hallmark of solid tumors—clinical perspectives. Cell Oncol (Dordr). 2021;44(4):715–37.
Li X, Zhou J, Wang X, Li C, Ma Z, Wan Q, Peng F. New advances in the research of clinical treatment and novel anticancer agents in tumor angiogenesis. Biomed Pharmacother. 2023;163:114806.
Abramson LP, Grundy PE, Rademaker AW, Helenowski I, Cornwell M, Katzenstein HM, Reynolds M, Arensman RM, Crawford SE. Increased microvascular density predicts relapse in Wilms’ tumor. J Pediatr Surg. 2003;38(3):325.
Ozluk Y, Kilicaslan I, Gulluoglu MG, Ayan I, Uysal V. The prognostic significance of angiogenesis and the effect of vascular endothelial growth factor on angiogenic process in Wilms’ tumour. Pathology. 2006;38(5):408–14.
Li W, Kessler P, Yeger H, Alami J, Reeve AE, Heathcott R, Skeen J, Williams BRG. A gene expression signature for relapse of primary Wilms tumors. Can Res. 2005;65(7):2592–601.
Frischer JS, Huang J, Serur A, Kadenhe-Chiweshe A, McCrudden KW, O’Toole K, Holash J, Yancopoulos GD, Yamashiro DJ, Kandel JJ. Effects of potent VEGF blockade on experimental Wilms tumor and its persisting vasculature. Int J Oncol. 2004;25(3):549–53.
Ferrara N, Hillan KJ, Gerber H-P, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3(5):391–400.
Kumar S, Burney IA, Al-Moundhri MS. Near complete resolution of refractory, relapsed, metastatic Wilms’ tumour in an adolescent with bevacizumab. J Coll Phys Surg-Pak. 2014;24(Suppl 1):S71–2.
Schiavetti A, Varrasso G, Collini P, Clerico A. Vincristine, irinotecan, and bevacizumab in relapsed Wilms tumor with diffuse anaplasia. J Pediatr Hematol Oncol. 2018;40(4):331–3.
Interiano RB, McCarville MB, Wu J, Davidoff AM, Sandoval J, Navid F. Pneumothorax as a complication of combination antiangiogenic therapy in children and young adults with refractory/recurrent solid tumors. J Pediatr Surg. 2015;50(9):1484–9.
Ostman A. PDGF receptors-mediators of autocrine tumor growth and regulators of tumor vasculature and stroma. Cytokine Growth Factor Rev. 2004;15(4):275–86.
Apte SM, Fan D, Killion JJ, Fidler IJ. Targeting the platelet-derived growth factor receptor in antivascular therapy for human ovarian carcinoma. Clin Cancer Res. 2004;10(3):897–908.
ManzatSaplacan RM, Balacescu L, Gherman C, Chira RI, Craiu A, Mircea PA, Lisencu C, Balacescu O. The role of PDGFs and PDGFRs in colorectal cancer. Mediat Inflamm. 2017;2017:4708076.
Corvigno S, Frödin M, Wisman GBA, Nijman HW, Van der Zee AG, Jirström K, Nodin B, Hrynchyk I, Edler D, Ragnhammar P, Johansson M, Dahlstrand H, Mezheyeuski A, Östman A. Multi-parametric profiling of renal cell, colorectal, and ovarian cancer identifies tumour-type-specific stroma phenotypes and a novel vascular biomarker. J Pathol Clin Res. 2017;3(3):214–24.
Huang J, Soffer SZ, Kim ES, McCrudden KW, Huang J, New T, Manley CA, Middlesworth W, O’Toole K, Yamashiro DJ, Kandel JJ. Vascular remodeling marks tumors that recur during chronic suppression of angiogenesis. Mol Cancer Res. 2004;2(1):36–42.
Aye JM, Stafman LL, Williams AP, Garner EF, Stewart JE, Anderson JC, Mruthyunjayappa S, Waldrop MG, Goolsby CD, Markert HR, Quinn C, Marayati R, Mroczek-Musulman E, Willey CD, Yoon KJ, Whelan KF, Beierle EA. The effects of focal adhesion kinase and platelet-derived growth factor receptor beta inhibition in a patient-derived xenograft model of primary and metastatic Wilms tumor. Oncotarget. 2019;10(53):5534–48.
Huang XL, Khan MI, Wang J, Ali R, Ali SW, Zahra Q-U-A, Kazmi A, Lolai A, Huang YL, Hussain A, Bilal M, Li F, Qiu B. Role of receptor tyrosine kinases mediated signal transduction pathways in tumor growth and angiogenesis—new insight and futuristic vision. Int J Biol Macromol. 2021;180:739–52.
Wang H, Yin Y, Li W, Zhao X, Yu Y, Zhu J, Qin Z, Wang Q, Wang K, Lu W, Liu J, Huang L. Over-expression of PDGFR-β promotes PDGF-induced proliferation, migration, and angiogenesis of EPCs through PI3K/Akt signaling pathway. PLoS ONE. 2012;7(2):e30503.
Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N, Yi P, Tang L, Pan Q, Rao S, Liang J, Tang Y, Su M, Luo X, Yang Y, Shi Y, Wang H, Zhou Y, Liao Q. The cancer metabolic reprogramming and immune response. Mol Cancer. 2021;20(1):28.
Guo J-Q, Wang C-D, Tang H-Y, Sang B-T, Liu X, Yi F-P, Wu X-M. PDGF-BB/PDGFRβ promotes epithelial-mesenchymal transition by affecting PI3K/AKT/mTOR-driven aerobic glycolysis in Wilms’ tumor G401 cells. Cell Biol Int. 2022;46(6):907–21.
Itatani Y, Kawada K, Yamamoto T, Sakai Y. Resistance to anti-angiogenic therapy in cancer-alterations to anti-VEGF pathway. Int J Mol Sci. 2018;19(4):1232.
Huang M, Lin Y, Wang C, Deng L, Chen M, Assaraf YG, Chen Z-S, Ye W, Zhang D. New insights into antiangiogenic therapy resistance in cancer: mechanisms and therapeutic aspects. Drug Resist Updates. 2022;64:100849.
Groenendijk A, Spreafico F, de Krijger RR, Drost J, Brok J, Perotti D, van Tinteren H, Venkatramani R, Godziński J, Rübe C, Geller JI, Graf N, van den Heuvel-Eibrink MM, Mavinkurve-Groothuis AMC. Prognostic factors for wilms tumor recurrence: A review of the literature. Cancers. 2021;13(13):3142.
Spini A, Ciccone V, Rosellini P, Ziche M, Lucenteforte E, Salvo F, Donnini S. Safety of anti-angiogenic drugs in pediatric patients with solid tumors: A systematic review and meta-analysis. Cancers. 2022;14(21):5315.
Rowe DH, Kayton ML, O’Toole KM, Ingram M, Stolar CJ, Kandel JJ. Pathological angiogenesis in a murine model of human Wilms’ tumor. J Pediatr Surg. 1999;34(5):676–9.
Sköldenberg EG, Christiansson J, Sandstedt B, Larsson A, Läckgren G, Christofferson R. Angiogenesis and angiogenic growth factors in Wilms tumor. J Urol. 2001;165(6 Pt 2):2274–9.
Wang J, Fan S, Feng Y, Zhang H, Zou W, Hu C. Antiangiogenic therapy for Wilms tumor in an adult and literature review. Anticancer Drugs. 2019;30(6):640–5.
Huang J, Moore J, Soffer S, Kim E, Rowe D, Manley CA, O’Toole K, Middlesworth W, Stolar C, Yamashiro D, Kandel J. Highly specific antiangiogenic therapy is effective in suppressing growth of experimental Wilms tumors. J Pediatr Surg. 2001;36(2):357–61.
Ghanem MA, van der Kwast TH, Molenaar WM, Safan MA, Nijman RJ, van Steenbrugge GJ. The predictive value of immunohistochemical markers in untreated Wilms’ tumour: are they useful? World J Urol. 2013;31(4):811–6.
Ghanem M, Nijman R, Safan M, van der Kwast T, Vansteenbrugge G. Expression and prognostic value of platelet-derived growth factor-AA and its receptor α in nephroblastoma. BJU Int. 2010;106(9):1389–93.
Thies KA, Hammer AM, Hildreth BE, Steck SA, Spehar JM, Kladney RD, Geisler JA, Das M, Russell LO, Bey JF, Bolyard CM, Pilarski R, Trimboli AJ, Cuitiño MC, Koivisto CS, Stover DG, Schoenfield L, Otero J, Godbout JP, Chakravarti A, Ringel MD, Ramaswamy B, Li Z, Kaur B, Leone G, Ostrowski MC, Sizemore ST, Sizemore GM. Stromal platelet-derived growth factor receptor-β signaling promotes breast cancer metastasis in the brain. Can Res. 2021;81(3):606–18.
Guérit E, Arts F, Dachy G, Boulouadnine B, Demoulin J-B. PDGF receptor mutations in human diseases. Cell Mol Life Sci. 2021;78(8):3867–81.
Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4(11):891–9.
de la Cruz-López KG, Castro-Muñoz LJ, Reyes-Hernández DO, García-Carrancá A, Manzo-Merino J. Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front Oncol. 2019;9:1143.
Azoitei N, Becher A, Steinestel K, Rouhi A, Diepold K, Genze F, Simmet T, Seufferlein T. PKM2 promotes tumor angiogenesis by regulating HIF-1α through NF-κB activation. Mol Cancer. 2016;15:3.
Wright SCE, Vasilevski N, Serra V, Rodon J, Eichhorn PJA. Mechanisms of resistance to PI3K inhibitors in cancer: Adaptive responses, drug tolerance and cellular plasticity. Cancers. 2021;13(7):1538.
Park JH, Pyun WY, Park HW. Cancer metabolism: phenotype, signaling and therapeutic targets. Cells. 2020;9(10):2308.
Courtnay R, Ngo DC, Malik N, Ververis K, Tortorella SM, Karagiannis TC. Cancer metabolism and the Warburg effect: the role of HIF-1 and PI3K. Mol Biol Rep. 2015;42(4):841–51.
Funding
Program for Youth Innovation in Future Medicine, Chongqing Medical University.
Author information
Authors and Affiliations
Contributions
BS was responsible for the experimental design of the study; Experimental operation; Results analysis and paper writing. CW and XL were responsible for the experimental design of the study. JG was responsible for the results analysis. JL was responsible for the bioinformatics analysis. XW was responsible for the experimental design and results analysis of the study.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sang, Bt., Wang, Cd., Liu, X. et al. PDGF-BB/PDGFRβ induces tumour angiogenesis via enhancing PKM2 mediated by the PI3K/AKT pathway in Wilms’ tumour. Med Oncol 40, 240 (2023). https://doi.org/10.1007/s12032-023-02115-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02115-5