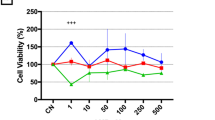
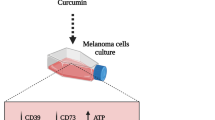
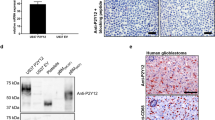
Abstract
ATP and adenosine (ADO) are critical players in the context of cancer. In the tumor microenvironment, the signaling dependent on these molecules, and immune cells, is regulated by an enzymatic chain and purinergic receptors called purinome. Primarily, the A2A receptor (A2AR) has a pro-tumor action since it reduces the immune response and favors the growth of malignant melanoma. Therefore, this study aimed to verify the effects of A2AR antagonism with Istradefylline (IST) on the purinergic signaling profile of the melanoma tumor and immunological compartments. We observed reduced tumor growth of melanoma in IST-treated animals. IST inhibited AKT/mTOR pathway, which is involved with tumor growth. In the tumor, spleen, and thymus, the modulation of purinergic enzymes (CD39, CD73, and E-ADA) characterized a pro-inflammatory profile since it favored increased extracellular concentrations of ATP to the detriment of ADO. A2AR inhibition generated a compensatory feedback process with increased A2AR expression at the tumor level. However, there was also an increase in the expression of the P2X7 receptor (P2X7R), which culminated in an increase in pro-inflammatory pathways with the release of IL-1β and pro-inflammatory cytokines such as IFN-γ and TNF-α. Our data evidence the cross-involvement between expression and action of the A2AR and P2X7R. We suggest that IST is a promising drug for off-label use in cancer since it promotes an anti-tumoral response by producing pro-inflammatory cytokines and blocking of AKT/mTOR tumor growth pathway.





Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Di Virgilio F, Adinolfi E. Extracellular purines, purinergic receptors and tumor growth. Oncog Nat Publ Gr. 2017;36:293–303.
Li X-Y, Moesta AK, Xiao C, Nakamura K, Casey M, Zhang H, et al. Targeting CD39 in cancer reveals an extracellular ATP- and inflammasome-driven tumor immunity. Cancer Discov. 2019;9:1754–73. https://doi.org/10.1158/2159-8290.CD-19-0541.
Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD 39 and CD 73: novel checkpoint inhibitor targets. Immunol Rev. 2017;276:121–44.
Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79:463–84. https://doi.org/10.1046/j.1471-4159.2001.00607.x.
Allard B, Allard D, Buisseret L, Stagg J. The adenosine pathway in immuno-oncology. Nat Rev Clin Oncol. 2020;17:611–29. https://doi.org/10.1038/s41571-020-0382-2.
Müller CE, Jacobson KA. Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim Biophys Acta—Biomembr. 2011;1808:1290–308.
Merighi S, Gessi S, Borea PA. Adenosine receptors: structure, distribution, and signal transduction. In: Borea PA, Varani K, Gessi S, Merighi S, Vincenzi F, editors. Adenosine recept. Cham: Springer; 2018. p. 33–57.
Cekic C, Day Y-J, Sag D, Linden J. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res AACR. 2014;74:7250–9.
Ohta A, Kini R, Ohta A, Subramanian M, Madasu M, Sitkovsky M. The development and immunosuppressive functions of CD4+ CD25+ FoxP3+ regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front Immunol. 2012;3:190.
Ma X-L, Shen M-N, Hu B, Wang B-L, Yang W-J, Lv L-H, et al. CD73 promotes hepatocellular carcinoma progression and metastasis via activating PI3K/AKT signaling by inducing Rap1-mediated membrane localization of P110β and predicts poor prognosis. J Hematol Oncol. 2019;12:1–17.
Zhou JZ, Riquelme MA, Gao X, Ellies LG, Sun L-Z, Jiang JX. Differential impact of adenosine nucleotides released by osteocytes on breast cancer growth and bone metastasis. Oncog Nat. 2015;34:1831–42.
Dungo R, Deeks ED. Istradefylline: first global approval. Drugs. 2013;73:875–82.
Hauser RA, Olanow CW, Kieburtz KD, Pourcher E, Docu-Axelerad A, Lew M, et al. Tozadenant (SYN115) in patients with Parkinson’s disease who have motor fluctuations on levodopa: a phase 2b, double-blind, randomised trial. Lancet Neurol. 2014;13:767–76.
Bleickardt CJ, LaShomb AL, Merkel CE, Hodgson RA. Adenosine A2A receptor antagonists do not disrupt rodent prepulse inhibition: an improved side effect profile in the treatment of parkinson’s disease. Parkinson’s Dis. 2012. https://doi.org/10.1155/2012/591094.
Doleski PH, Cabral FL, Adefegha SA, Jantsch MH, Ebone RS, Leal DBR, et al. Distinct kinetics for nucleotide hydrolysis in lymphocytes isolated from blood, spleen and cervical lymph nodes: Characterization of ectonucleotidase activity. Cell Biochem Funct. 2021;39:511–20.
Tan YS, Lei YL. Isolation of tumor-infiltrating lymphocytes by ficoll-paque density gradient centrifugation. In: Allen IC, editor. Mouse model innate immun methods protoc. Springer: New York; 2019. p. 93–9.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Giusti GGB. Adenosine deaminase. Methods Enzym Anal. 1984. https://doi.org/10.1016/B978-0-12-091302-2.50108-0.
Chan KM, Delfert D, Junger KD. A direct colorimetric assay for Ca2+-stimulated ATPase activity. Anal Biochem. 1986;157:375–80.
Morgan E, Varro R, Sepulveda H, Ember JA, Apgar J, Wilson J, et al. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin Immunol. 2004;110:252–66.
da Silva JLG, Viana AR, Passos DF, Krause LMF, Miron VV, Schetinger MRC, et al. Istradefylline modulates purinergic enzymes and reduces malignancy-associated factors in B16F10 melanoma cells. Purinergic Signal. 2022. https://doi.org/10.1007/s11302-022-09909-8.
Bronte V, Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity. 2013;39:806–18.
Kukulski F, Lévesque SA, Sévigny J. Chapter 9—Impact of ectoenzymes on P2 and P1 receptor signaling. In: Jacobson KA, Linden JBT-A, editors. Pharmacol purine pyrimidine recept. Cambridge: Academic Press; 2011. p. 263–99.
Pulte ED, Broekman MJ, Olson KE, Drosopoulos JHF, Kizer JR, Islam N, et al. CD39/NTPDase-1 activity and expression in normal leukocytes. Thromb Res. 2007;121:309–17.
Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11:201.
Lara R, Adinolfi E, Harwood CA, Philpott M, Barden JA, Di Virgilio F, et al. P2X7 in cancer: from molecular mechanisms to therapeutics. Front Pharmacol. 2020. https://doi.org/10.3389/fphar.2020.00793.
Congreve M, Brown GA, Borodovsky A, Lamb ML. Targeting adenosine A2A receptor antagonism for treatment of cancer. Expert Opin Drug Discov. 2018;13:997–1003. https://doi.org/10.1080/17460441.2018.1534825.
De Marchi E, Pegoraro A, Turiello R, Di Virgilio F, Morello S, Adinolfi E. A2A Receptor contributes to tumor progression in P2X7 null mice. bioRxiv. 2022. https://doi.org/10.3389/fcell.2022.876510.
Orioli E, De Marchi E, Lisa Giuliani A, Adinolfi E. P2X7 receptor orchestrates multiple signalling pathways triggering inflammation, autophagy and metabolic/trophic responses. Curr Med Chem. 2017;24:2261–75.
Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–91.
Speiser DE, Ho P-C, Verdeil G. Regulatory circuits of T cell function in cancer. Nat Rev Immunol. 2016;16:599–611. https://doi.org/10.1038/nri.2016.80.
Beavis PA, Divisekera U, Paget C, Chow MT, John LB, Devaud C, et al. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc Natl Acad Sci USA. 2013;110:14711–6.
Chan SH, Perussia B, Gupta JW, Kobayashi M, Pospísil M, Young HA, et al. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991;173:869–79. https://doi.org/10.1084/jem.173.4.869.
Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S, et al. IL-10 elicits IFNγ-dependent tumor immune surveillance. Cancer Cell. 2011;20:781–96.
Fujii S, Shimizu K, Shimizu T, Lotze MT. Interleukin-10 promotes the maintenance of antitumor CD8+ T-cell effector function in situ. Blood. 2001;98:2143–51. https://doi.org/10.1182/blood.V98.7.2143.
Punt S, Langenhoff JM, Putter H, Fleuren GJ, Gorter A, Jordanova ES. The correlations between IL-17 vs. Th17 cells and cancer patient survival: a systematic review. Oncoimmunology. 2015;4:e984547. https://doi.org/10.4161/2162402X.2014.984547.
Kuen D-S, Kim B-S, Chung Y. IL-17-producing cells in tumor immunity: friends or foes? Immune Netw. 2020. https://doi.org/10.4110/in.2020.20.e6.
He G, Dhar D, Nakagawa H, Font-Burgada J, Ogata H, Jiang Y, et al. Identification of liver cancer progenitors whose malignant progression depends on autocrine IL-6 signaling. Cell. 2013;155:384–96.
Oh K, Lee O-Y, Shon SY, Nam O, Ryu PM, Seo MW, et al. A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast Cancer Res. 2013;15:R79. https://doi.org/10.1186/bcr3473.
Chang Q, Daly L, Bromberg J. The IL-6 feed-forward loop: a driver of tumorigenesis. Semin Immunol. 2014;26:48–53.
Di Virgilio F, Meyer BC, Greenberg S, Silverstein SC. Fc receptor-mediated phagocytosis occurs in macrophages at exceedingly low cytosolic Ca2+ levels. J Cell Biol. 1988;106:657–66. https://doi.org/10.1083/jcb.106.3.657.
Grassi F, De Ponte CB. The P2X7 receptor in tumor immunity. Front Cell Dev Biol. 2021. https://doi.org/10.3389/fcell.2021.694831.
Shao B-Z, Xu Z-Q, Han B-Z, Su D-F, Liu C. NLRP3 inflammasome and its inhibitors: a review. Front Pharmacol. 2015. https://doi.org/10.3389/fphar.2015.00262.
Shi L, Wu Z, Miao J, Du S, Ai S, Xu E, et al. Adenosine interaction with adenosine receptor A2a promotes gastric cancer metastasis by enhancing PI3K–AKT–mTOR signaling. Mol Biol Cell. 2019;30:2527–34. https://doi.org/10.1091/mbc.E19-03-0136.
Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, immunity, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–75. https://doi.org/10.1146/annurev.cellbio.17.1.615.
Xu S, Yang Z, Jin P, Yang X, Li X, Wei X, et al. Metformin suppresses tumor progression by inactivating stromal fibroblasts in ovarian cancer. Mol Cancer Ther. 2018;17:1291–302. https://doi.org/10.1158/1535-7163.MCT-17-0927.
Yasuda T. Hyaluronan inhibits Akt, leading to nuclear factor-<I>κ</I>B down-regulation in lipopolysaccharide-stimulated U937 macrophages. J Pharmacol Sci. 2011;115:509–15.
Tazzari PL, Cappellini A, Ricci F, Evangelisti C, Papa V, Grafone T, et al. Multidrug resistance-associated protein 1 expression is under the control of the phosphoinositide 3 kinase/Akt signal transduction network in human acute myelogenous leukemia blasts. Leukemia. 2007;21:427–38. https://doi.org/10.1038/sj.leu.2404523.
Usman MW, Gao J, Zheng T, Rui C, Li T, Bian X, et al. Macrophages confer resistance to PI3K inhibitor GDC-0941 in breast cancer through the activation of NF-κB signaling. Cell Death Dis. 2018;9:809. https://doi.org/10.1038/s41419-018-0849-6.
Giacomelli C, Daniele S, Romei C, Tavanti L, Neri T, Piano I, et al. The A2B adenosine receptor modulates the epithelial—mesenchymal transition through the balance of cAMP/PKA and MAPK/ERK pathway activation in human epithelial lung cells. Front Pharmacol. 2018. https://doi.org/10.3389/fphar.2018.00054.
Gessi S, Bencivenni S, Battistello E, Vincenzi F, Colotta V, Catarzi D, et al. Inhibition of A2A adenosine receptor signaling in cancer cells proliferation by the novel antagonist TP455. Front Pharmacol. 2017;8:888.
Bergholz JS, Zhao JJ. How compensatory mechanisms and adaptive rewiring have shaped our understanding of therapeutic resistance in cancer. Cancer Res. 2021;81:6074–7. https://doi.org/10.1158/0008-5472.CAN-21-3605.
Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–74. https://doi.org/10.1172/JCI34739.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) -Finance Code 001, Grant Number 88887.338883/2019-00.
Author information
Authors and Affiliations
Contributions
Conceptualization: JLGdS, DBRL. Data curation: JLGdS, formal analysis JLGdS, DFP; investigation: JLGdS, VVM, RSE1. methodology: JLGdS, VVM, FLC, AAC1, CHDP1, COG1, project administration: JLGdS, DBRL; resources: DBRL, MRCS, supervision: DBRL, validation: DBRL, MRCS, visualization: JLGdS, DFP; roles/writing—original draf: JLGdS; writing—review & editing: JLGdS, DFP. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no conflicts of interest to declare.
Ethical Approval
This project was approved by the Animals Use Ethics Commission of Universidade Federal de Santa Maria (CEUA/UFSM). The approval was granted under protocol number 4748290420.
Consent for publication
All authors have seen and agreed with the content and given consent to submit the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gutknecht da Silva, J.L., Passos, D.F., Cabral, F.L. et al. Istradefylline induces A2A/P2X7 crosstalk expression inducing pro-inflammatory signal, and reduces AKT/mTOR signaling in melanoma-bearing mice. Med Oncol 40, 178 (2023). https://doi.org/10.1007/s12032-023-02033-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02033-6