Abstract
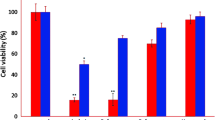
Oral squamous cell carcinoma (OSCC), main head and neck squamous cell carcinomas (HNSCCs), remains a global health concern with unknown pathogenesis. Veillonella parvula NCTC11810 was observed to decrease in saliva microbiome of OSCC patients in this study and the aim was to detect the novel role of Veillonella parvula NCTC11810 in regulating the biological characteristics of OSCC through TROP2/PI3K/Akt pathway. Oral microbial community changes of OSCC patients were detected by 16S rDNA gene sequencing technology. CCK8 assay, Transwell assay, and Annexin V-FITC/PI staining were used for proliferation, invasion, and apoptosis analysis of OSCC cell lines. Expression of proteins were determined by Western blotting analysis. Veillonella parvula NCTC11810 showed decreased in saliva microbiome of TROP2 high-expressed OSCC patients. Culture supernatant of Veillonella parvula NCTC11810 promoted the apoptosis and inhibited the proliferation and invasion ability of HN6 cells, while sodium propionate (SP), the main metabolite of Veillonella parvula NCTC11810, played a similar role through the inhibition of TROP2/PI3K/Akt pathway. Studies above supported the proliferation-inhibiting, invasion-inhibiting, and apoptosis-promoting function of Veillonella parvula NCTC11810 in OSCC cells which provided new insights into oral microbiota and their metabolite as a therapeutic method for OSCC patients with TROP2 high expressing.







Similar content being viewed by others
References
Tang G, et al. TROP2 increases growth and metastasis of human oral squamous cell carcinoma through activation of the PI3K/Akt signaling pathway[J]. Int J Mol Med. 2019;44(6):2161–70.
Wei J, et al. Salvianolic acid B inhibits glycolysis in oral squamous cell carcinoma via targeting PI3K/AKT/HIF-1α signaling pathway[J]. Cell Death Dis. 2018;9(6):599.
Roi A, et al. The challenges of OSCC diagnosis: salivary cytokines as potential biomarkers[J]. J Clin Med. 2020;9(9):2866.
Jia L, et al. Trop2 inhibition of P16 expression and the cell cycle promotes intracellular calcium release in OSCC[J]. Int J Biol Macromol. 2020;164:2409–17.
Sasahira T, Bosserhoff AK, Kirita T. The importance of melanoma inhibitory activity gene family in the tumor progression of oral cancer[J]. Pathol Int. 2018;68(5):278–86.
Lipinski M, et al. Human trophoblast cell-surface antigens defined by monoclonal antibodies[J]. Proc Natl Acad Sci USA. 1981;78:5147–50.
Lenárt S, et al. Trop2: jack of all trades, master of none[J]. Cancers (Basel). 2020;12(11):3328.
Stewart D, Cristea M. Antibody-drug conjugates for ovarian cancer: current clinical development[J]. Curr Opin Obstet Gynecol. 2019;31(1):18–23.
Liu J, et al. A novel human monoclonal Trop2-IgG antibody inhibits ovarian cancer growth in vitro and in vivo[J]. Biochem Biophys Res Commun. 2019;512(2):276–82.
Zhao W, et al. The role and molecular mechanism of Trop2 induced epithelial-mesenchymal transition through mediated β-catenin in gastric cancer[J]. Cancer Med. 2019;8(3):1135–47.
Zhao W, et al. The killing effect of novel bi-specific Trop2/PD-L1 CAR-T cell targeted gastric cance[J]. Am J Cancer Res. 2019;9(8):1846–56.
Jordheim LP, et al. Unexpected growth-promoting effect of Oxaliplatin in excision repair cross-complementation group 1 transfected human colon cancer cells[J]. Pharmacology. 2018;102(3–4):161–8.
Nishimura T, et al. Photoimmunotherapy targeting biliary-pancreatic cancer with humanized anti-TROP2 antibody[J]. Cancer Med. 2019;8(18):7781–92.
Wang XD, et al. Trop2 inhibition suppresses the proliferation and invasion of laryngeal carcinoma cells via the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway[J]. Mol Med Rep. 2015;12(1):865–70.
Wanger TM, et al. Differential regulation of TROP2 release by PKC isoforms through vesicles and ADAM17[J]. Cell Signal. 2015;27(7):1325–35.
Zhang B, et al. Tissue mechanics and expression of TROP2 in oral squamous cell carcinoma with varying differentiation[J]. BMC Cancer. 2020;20(1):815.
Peterson J, et al. The NIH Human Microbiome Project[J]. Genome Res. 2009;19(12):2317–23.
Zhou CB, Zhou YL, Fang JY. Gut microbiota in cancer immune response and immunotherapy[J]. Trends Cancer. 2021;7(7):647–60.
Gao R, et al. Gut microbiota and colorectal cancer[J]. Eur J Clin Microbiol Infect Dis. 2017;36(5):757–69.
Angelucci F, et al. Antibiotics, gut microbiota, and Alzheimer’s disease[J]. J Neuroinflammation. 2019;16(1):108.
Zhao H, et al. Variations in oral microbiota associated with oral cancer[J]. Sci Rep. 2017;7(1):11773.
Mager DL, et al. The salivary microbiota as a diagnostic indicator of oral cancer: a descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects[J]. J Transl Med. 2005;3:27.
Wang L, et al. Variations in oral microbiota composition are associated with a risk of throat cancer[J]. Front Cell Infect Microbiol. 2019;9:205.
Börnigen D, et al. Alterations in oral bacterial communities are associated with risk factors for oral and oropharyngeal cancer[J]. Sci Rep. 2017;7(1):17686.
Granato DC, et al. Meta-omics analysis indicates the saliva microbiome and its proteins associated with the prognosis of oral cancer patients[J]. Biochim Biophys Acta Proteins Proteom. 2021;1869(8):140659.
Heintz-Buschart A, Wilmes P. Human gut microbiome: function matters[J]. Trends Microbiol. 2018;26(7):563–74.
Han JH, et al. The effects of propionate and valerate on insulin responsiveness for glucose uptake in 3T3-L1 adipocytes and C2C12 myotubes via G protein-coupled receptor 41[J]. PLoS One. 2014;9(4):e95268.
Bindels LB, et al. Gut microbiota-derived propionate reduces cancer cell proliferation in the liver[J]. Br J Cancer. 2012;107(8):1337–44.
Zeng H, et al. Superior inhibitory efficacy of butyrate over propionate and acetate against human colon cancer cell proliferation via cell cycle arrest and apoptosis: linking dietary fiber to cancer prevention[J]. Nutr Res. 2020;83:63–72.
Thirunavukkarasan M, et al. Short-chain fatty acid receptors inhibit invasive phenotypes in breast cancer cells[J]. PLoS One. 2017;12(10):e0186334.
Sivaprakasam S, Prasad PD, Singh N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis[J]. Pharmacol Ther. 2016;164:144–51.
Tang G, et al. High expression of TROP2 is correlated with poor prognosis of oral squamous cell carcinoma[J]. Pathol Res Pract. 2018;214(10):1606–12.
Sherrard LJ, Bell SC, Tunney MM. The role of anaerobic bacteria in the cystic fibrosis airway[J]. Curr Opin Pulm Med. 2016;22(6):637–43.
Brook I. Veillonella infections in children[J]. J Clin Microbiol. 1996;34(5):1283–5.
Luo YX, et al. Research progress in the relationship between Veillonella and oral diseases[J]. Hua Xi Kou Qiang Yi Xue Za Zhi. 2020;38(5):576–82.
Jia YJ, et al. Association between oral microbiota and cigarette smoking in the chinese population[J]. Front Cell Infect Microbiol. 2021;11:658203.
Luppens SB, et al. Effect of Veillonella parvula on the antimicrobial resistance and gene expression of Streptococcus mutans grown in a dual-species biofilm[J]. Oral Microbiol Immunol. 2008;23(3):183–9.
Bajic D, et al. Gut microbiota-derived propionate regulates the expression of reg3 mucosal lectins and ameliorates experimental colitis in mice[J]. J Crohns Colitis. 2020;14(10):1462–72.
Høgh RI, et al. Metabolism of short-chain fatty acid propionate induces surface expression of NKG2D ligands on cancer cells[J]. Faseb J. 2020;34(11):15531–46.
Gu QZ, et al. TROP2 promotes cell proliferation and migration in osteosarcoma through PI3K/AKT signaling[J]. Mol Med Rep. 2018;18(2):1782–8.
Li X, et al. TROP2 promotes proliferation, migration and metastasis of gallbladder cancer cells by regulating PI3K/AKT pathway and inducing EMT[J]. Oncotarget. 2017;8(29):47052–63.
Rivera C. Essentials of oral cancer[J]. Int J Clin Exp Pathol. 2015;8(9):11884–94.
Fong D, et al. TROP2: a novel prognostic marker in squamous cell carcinoma of the oral cavity[J]. Mod Pathol. 2008;21(2):186–91.
Guerra E, et al. The Trop-2 signalling network in cancer growth. Oncogene. 2013;32(12):1594–600.
Ambrogi F, et al. Trop-2 is a determinant of breast cancer survival[J]. PLoS One. 2014;9(5):e96993.
Cubas R, et al. Trop2 expression contributes to tumor pathogenesis by activating the ERK MAPK pathway[J]. Mol Cancer. 2010;9:253.
Almeida LY, et al. FASN inhibition sensitizes metastatic OSCC cells to cisplatin and paclitaxel by downregulating cyclin B1[J]. Oral Dis. 2023;29(2):649–60.
Meng X, et al. The role of non-coding RNAs in drug resistance of oral squamous cell carcinoma and therapeutic potential[J]. Cancer Commun (Lond). 2021;41(10):981–1006.
Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota[J]. Environ Microbiol. 2017;19(1):29–41.
Molinaro A, et al. Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nat Commun. 2020;11(1):5881.
Funding
This study was financially supported by the National Natural Science Foundation of China (81872426), the Nanjing Medical Science and Technology Development Fund (YKK21152), and the Science and Technology Development Fund of Nanjing Medical University (NMUB20210072).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12032_2023_1962_MOESM1_ESM.pdf
Supplementary file1 (PDF 102 KB)—The 16 S-V4 amplicon sequencing and nucleotide Blast program of clone 12 from Columbia blood plates. (A) The 16 SV4 amplicon sequence result of clone 12 picked out from Columbia blood plates; (B) The sequencing results showed that the nucleotide sequence presented a high percentage of similarity (99.71%) using the Nucleotide BLAST program with a peculiar microorganism Veillonella parvula NCTC11810
12032_2023_1962_MOESM3_ESM.pdf
Supplementary file3 (PDF 485 KB)—The western blotting result of cleaved GSDMD expression level in HN6 cells; Supernatant: culture supernatant of Veillonella parvula NCTC11810-treated group; SP-Treated: sodium propionate-treated group; Heated-V.P.: Heat-inactivated Veillonella parvula NCTC11810-treated group; V.P.: Veillonella parvula NCTC11810-treated group
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chang, X., Chen, Y., Cui, D. et al. Propionate-producing Veillonella parvula regulates the malignant properties of tumor cells of OSCC. Med Oncol 40, 98 (2023). https://doi.org/10.1007/s12032-023-01962-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-01962-6