Abstract
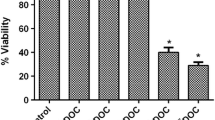
The present study aimed to assess the influence of ciprofloxacin (CIP) against the doxorubicin (DOX)-resistant androgen-independent prostate cancer DU145 cells. The DOX-resistant DU145 (DU145/DOX20) cells were established by exposing DU145 cells to the increasing concentrations of DOX. The antiproliferative effect of CIP was examined through employing MTT, colony formation, and 3D culture assays. DU145/DOX20 cells exhibited a twofold higher IC50 value for DOX, an increased ABCB1 transporter activity, and some morphological changes accompanied by a decrease in spheroid size, adhesive and migration potential compared to DU145 cells. CIP (5 and 25 µg mL−1) resulted in a higher reduction in the viability of DU145/DOX20 cells than in DU145 cells. DU145/DOX20 cells were more resistant to CIP in 3D culture compared to the 2D one. No spheroid formation was observed for DU145/DOX20 cells treated with DOX and CIP combination. CIP and DOX, alone or in combination, significantly reduced the growth of DU145 spheroids. CIP in combination with 20 nM DOX prevented the colony formation of DU145 cells. The clonogenicity of DU145/DOX20 cells could not be estimated due to their low adhesive potential. CIP alone caused a significant reduction in the migration of DU145 cells and resulted in a more severe decrease in the wound closure ability of DOX-exposed ones. We identified that CIP enhanced DOX sensitivity in DU145 and DU145/DOX20 cells. This study suggested the co-delivery of low concentrations of CIP and DOX may be a promising strategy in treating the DOX-resistant and -sensitive hormone-refractory prostate cancer.







Similar content being viewed by others
References
Chen J, Chen B, Zou Z, Li W, Zhang Y, Xie J, et al. Costunolide enhances doxorubicin-induced apoptosis in prostate cancer cells via activated mitogen-activated protein kinases and generation of reactive oxygen species. Oncotarget. 2017. https://doi.org/10.18632/oncotarget.22592.
Li S, Yuan S, Zhao Q, Wang B, Wang X, Li K. Quercetin enhances chemotherapeutic effect of doxorubicin against human breast cancer cells while reducing toxic side effects of it. Biomed Pharmacother. 2018. https://doi.org/10.1016/j.biopha.2018.02.055.
Bai T, Liu Y, Li B. LncRNA LOXL1-AS1/miR-let-7a-5p/EGFR-related pathway regulates the doxorubicin resistance of prostate cancer DU-145 cells. IUBMB Life. 2019. https://doi.org/10.1002/iub.2075.
Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013. https://doi.org/10.1111/j.2042-7158.2012.01567.x.
Zahreddine H, Borden K. Mechanisms and insights into drug resistance in cancer. Front Pharmacol. 2013. https://doi.org/10.3389/fphar.2013.00028.
Kim S-Y. Cancer energy metabolism: shutting power off cancer factory. Biomol Ther. 2018. https://doi.org/10.4062/biomolther.2017.184.
Shahruzaman SH, Fakurazi S, Maniam S. Targeting energy metabolism to eliminate cancer cells. Cancer Manag Res. 2018. https://doi.org/10.2147/CMAR.S167424.
Willcocks S, Huse KK, Stabler R, Oyston PC, Scott A, Atkins HS, et al. Genome-wide assessment of antimicrobial tolerance in Yersinia pseudotuberculosis under ciprofloxacin stress. Microb Genomics. 2019. https://doi.org/10.1099/mgen.0.000304.
Boerema J-BJ, Dalhoff A, Debruyne FM. Ciprofloxacin distribution in prostatic tissue and fluid following oral administration. Chemotherapy. 1985. https://doi.org/10.1159/000238308.
Hangas A, Aasumets K, Kekäläinen NJ, Paloheinä M, Pohjoismäki JL, Gerhold JM, et al. Ciprofloxacin impairs mitochondrial DNA replication initiation through inhibition of Topoisomerase 2. Nucleic Acids Res. 2018. https://doi.org/10.1093/nar/gky793.
Gürbay A, Hıncal F. Ciprofloxacin-induced glutathione redox status alterations in rat tissues. Drug Chem Toxicol. 2004. https://doi.org/10.1081/dct-120037504.
Bush NG, Diez-Santos I, Abbott LR, Maxwell A. Quinolones: mechanism, lethality and their contributions to antibiotic resistance. Molecules. 2020. https://doi.org/10.3390/molecules25235662.
Aranha O, Grignon R, Fernandes N, McDONNELL TJ, Wood DP, Sarkar FH. Suppression of human prostate cancer cell growth by ciprofloxacin is associated with cell cycle arrest and apoptosis. Int J Oncol. 2003. https://doi.org/10.3892/ijo.22.4.787.
Yadav V, Talwar P. Repositioning of fluoroquinolones from antibiotic to anti-cancer agents: an underestimated truth. Biomed Pharmacother. 2019. https://doi.org/10.1016/j.biopha.2018.12.119.
Kloskowski T, Szeliski K, Fekner Z, Rasmus M, Dąbrowski P, Wolska A, et al. Ciprofloxacin and levofloxacin as potential drugs in genitourinary cancer treatment—the effect of dose-response on 2D and 3D cell cultures. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms222111970.
Chrzanowska A, Olejarz W, Kubiak-Tomaszewska G, Ciechanowicz AK, Struga M. The effect of fatty acids on ciprofloxacin cytotoxic activity in prostate cancer cell lines—does lipid component enhance anticancer ciprofloxacin potential? Cancers. 2022. https://doi.org/10.3390/cancers14020409.
Gupta P, Gao H-L, Ashar YV, Karadkhelkar NM, Yoganathan S, Chen Z-S. Ciprofloxacin enhances the chemosensitivity of cancer cells to ABCB1 substrates. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20020268.
Pinto AC, Moreira JN, Simões S. Ciprofloxacin sensitizes hormone-refractory prostate cancer cell lines to doxorubicin and docetaxel treatment on a schedule-dependent manner. Cancer Chemother Pharmacol. 2009. https://doi.org/10.1007/s00280-008-0892-6.
Park MS, Okochi H, Benet LZ. Is ciprofloxacin a substrate of P-glycoprotein? Arch Drug Inf. 2011. https://doi.org/10.1111/j.1753-5174.2010.00032.x.
David-Beabes GL, Overman MJ, Petrofski JA, Campbell PA, de Marzo AM, Nelson WG. Doxorubicin-resistant variants of human prostate cancer cell lines DU 145, PC-3, PPC-1, and TSU-PR1: characterization of biochemical determinants of antineoplastic drug sensitivity. Int J Oncol. 2000. https://doi.org/10.3892/ijo.17.6.1077.
Langdon SP. Cancer cell culture. Totawa: Humana Press Inc; 2010.
Wang W, Wang L, Mizokami A, Shi J, Zou C, Dai J, et al. Down-regulation of E-cadherin enhances prostate cancer chemoresistance via Notch signaling. Chin J Cancer. 2017. https://doi.org/10.1186/s40880-017-0203-x.
Munshi A, Hobbs M, Meyn RE. Clonogenic cell survival assay. Totawa: Humana Press Inc; 2005. https://doi.org/10.1385/1-59259-869-2:021.
Aldaghi SA, Jalal R. Concentration-dependent dual effects of ciprofloxacin on SB-590885-resistant BRAFV600E A375 melanoma cells. Chem Res Toxicol. 2019. https://doi.org/10.1021/acs.chemrestox.8b00335.
Li Y, Wang M, Zhi P, You J, Gao J-Q. Metformin synergistically suppress tumor growth with doxorubicin and reverse drug resistance by inhibiting the expression and function of P-glycoprotein in MCF7/ADR cells and xenograft models. Oncotarget. 2018. https://doi.org/10.18632/oncotarget.23187.
Ciarimboli G. Introduction to the cellular transport of organic cations. In: Ciarimboli G, Gautron S, Schlatter E, editors. Organic cation transporters. Cham: Springer; 2016. https://doi.org/10.1007/978-3-319-23793-0_1.
Zupkó I, Molnár J, Réthy B, Minorics R, Frank É, Wölfling J, et al. Anticancer and multidrug resistance-reversal effects of solanidine analogs synthetized from pregnadienolone acetate. Molecules. 2014. https://doi.org/10.3390/molecules19022061.
Sims JT, Ganguly SS, Bennett H, Friend JW, Tepe J, Plattner R. Imatinib reverses doxorubicin resistance by affecting activation of STAT3-dependent NF-κB and HSP27/p38/AKT pathways and by inhibiting ABCB1. PLoS ONE. 2013. https://doi.org/10.1371/journal.pone.0055509.
Boesch M, Wolf D, Sopper S. Optimized stem cell detection using the DyeCycle-triggered side population phenotype. Stem Cells Int. 2016. https://doi.org/10.1155/2016/1652389.
Jouan E, Le Vee M, Denizot C, Da Violante G, Fardel O. The mitochondrial fluorescent dye rhodamine 123 is a high-affinity substrate for organic cation transporters (OCT s) 1 and 2. Fundam Clin Pharmacol. 2014. https://doi.org/10.1111/j.1472-8206.2012.01071.x.
Marquez B, Ameye G, Vallet CM, Tulkens PM, Poirel HA, Van Bambeke F. Characterization of Abcc4 gene amplification in stepwise-selected mouse J774 macrophages resistant to the topoisomerase II inhibitor ciprofloxacin. PLoS ONE. 2011. https://doi.org/10.1371/journal.pone.0028368.
Wang D, Wang J, Zhang J, Yi X, Piao J, Li L, et al. Decrease of ABCB1 protein expression and increase of G1 phase arrest induced by oleanolic acid in human multidrug-resistant cancer cells. Exp Ther Med. 2021. https://doi.org/10.3892/etm.2021.10167.
Bray J, Sludden J, Griffin M, Cole M, Verrill M, Jamieson D, et al. Influence of pharmacogenetics on response and toxicity in breast cancer patients treated with doxorubicin and cyclophosphamide. Br J Cancer. 2010. https://doi.org/10.1038/sj.bjc.6605587.
Li Z, Chen C, Chen L, Hu D, Yang X, Zhuo W, et al. STAT5a confers doxorubicin resistance to breast cancer by regulating ABCB1. Front Oncol. 2021. https://doi.org/10.3389/fonc.2021.697950.
Phiboonchaiyanan PP, Kiratipaiboon C, Chanvorachote P. Ciprofloxacin mediates cancer stem cell phenotypes in lung cancer cells through caveolin-1-dependent mechanism. Chem Biol Interact. 2016. https://doi.org/10.1016/j.cbi.2016.03.005.
Lin M-C, Huang M-J, Liu C-H, Yang T-L, Huang M-C. GALNT2 enhances migration and invasion of oral squamous cell carcinoma by regulating EGFR glycosylation and activity. Oral Oncol. 2014. https://doi.org/10.1016/j.oraloncology.2014.02.003.
Heger JI, Froehlich K, Pastuschek J, Schmidt A, Baer C, Mrowka R, et al. Human serum alters cell culture behavior and improves spheroid formation in comparison to fetal bovine serum. Exp Cell Res. 2018. https://doi.org/10.1016/j.yexcr.2018.02.017.
Takeda N, Kondo M, Ito S, Ito Y, Shimokata K, Kume H. Role of RhoA inactivation in reduced cell proliferation of human airway smooth muscle by simvastatin. Am J Respir Cell Mol Biol. 2006. https://doi.org/10.1165/rcmb.2006-0034OC.
Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2012. https://doi.org/10.1016/j.addr.2012.09.027.
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial–mesenchymal transitions in development and disease. Cell. 2009. https://doi.org/10.1016/j.cell.2009.11.007.
Shankaranarayanan JS, Kanwar JR, Al-Juhaishi AJA, Kanwar RK. Doxorubicin conjugated to immunomodulatory anticancer lactoferrin displays improved cytotoxicity overcoming prostate cancer chemo resistance and inhibits tumour development in TRAMP mice. Sci Rep. 2016. https://doi.org/10.1038/srep32062.
Amend SR, Torga G, Lin KC, Kostecka LG, de Marzo A, Austin RH, et al. Polyploid giant cancer cells: unrecognized actuators of tumorigenesis, metastasis, and resistance. Prostate. 2019. https://doi.org/10.1002/pros.23877.
Pienta KJ, Hammarlund EU, Axelrod R, Brown JS, Amend SR. Poly-aneuploid cancer cells promote evolvability, generating lethal cancer. Evol Appl. 2020. https://doi.org/10.1111/eva.12929.
Herbein G, Nehme Z. Polyploid giant cancer cells, a hallmark of oncoviruses and a new therapeutic challenge. Front Oncol. 2020. https://doi.org/10.3389/fonc.2020.567116.
Mirzayans R, Andrais B, Murray D. Roles of polyploid/multinucleated giant cancer cells in metastasis and disease relapse following anticancer treatment. Cancers. 2018. https://doi.org/10.3390/cancers10040118.
Song Y, Zhao Y, Deng Z, Zhao R, Huang Q. Stress-induced polyploid giant cancer cells: unique way of formation and non-negligible characteristics. Front Oncol. 2021. https://doi.org/10.3389/fonc.2021.724781.
Gupta SK, Singh P, Ali V, Verma M. Role of membrane-embedded drug efflux ABC transporters in the cancer chemotherapy. Oncol Rev. 2020. https://doi.org/10.4081/oncol.2020.448.
Zhou Y, Tozzi F, Chen J, Fan F, Xia L, Wang J, et al. Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res. 2012. https://doi.org/10.1158/0008-5472.CAN-11-1674.
Kozieł R, Zabłocki K, Duszyński J. Calcium signals are affected by ciprofloxacin as a consequence of reduction of mitochondrial DNA content in Jurkat cells. Antimicrob Agents Chemother. 2006. https://doi.org/10.1128/AAC.50.5.1664-1671.2006.
Montani M, Herrmanns T, Müntener M, Wild P, Sulser T, Kristiansen G. Multidrug resistance protein 4 (MRP4) expression in prostate cancer is associated with androgen signaling and decreases with tumor progression. Virchows Arch. 2013. https://doi.org/10.1007/s00428-013-1429-x.
Davis R, Markham A, Balfour JA. Ciprofloxacin. Drugs. 1996. https://doi.org/10.2165/00003495-199651060-00010.
Boya P, Andreau K, Poncet D, Zamzami N, Perfettini J-L, Metivier D, et al. Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J Exp Med. 2003. https://doi.org/10.1084/jem.20021952.
Vallet CM, Marquez B, Ngabirano E, Lemaire S, Mingeot-Leclercq M-P, Tulkens PM, et al. Cellular accumulation of fluoroquinolones is not predictive of their intracellular activity: studies with gemifloxacin, moxifloxacin and ciprofloxacin in a pharmacokinetic/pharmacodynamic model of uninfected and infected macrophages. Int J Antimicrob Agents. 2011. https://doi.org/10.1016/j.ijantimicag.2011.05.011.
Calcagno AM, Fostel JM, To KK, Salcido CD, Martin SE, Chewning KJ, et al. Single-step doxorubicin-selected cancer cells overexpress the ABCG2 drug transporter through epigenetic changes. Br J Cancer. 2008. https://doi.org/10.1038/sj.bjc.6604334.
Ware JH, Zhou Z, Guan J, Kennedy AR, Kopelovich L. Establishment of human cancer cell clones with different characteristics: a model for screening chemopreventive agents. Anticancer Res. 2007;27:1–16.
Jeon JH, Kim DK, Shin Y, Kim HY, Song B, Lee EY, et al. Migration and invasion of drug-resistant lung adenocarcinoma cells are dependent on mitochondrial activity. Exp Mol Med. 2016. https://doi.org/10.1038/emm.2016.129.
Friedrich J, Ebner R, Kunz-Schughart LA. Experimental anti-tumor therapy in 3-D: spheroids–old hat or new challenge? Int J Radiat Biol. 2007. https://doi.org/10.1080/09553000701727531.
Shah A, Lettieri J, Nix D, Wilton J, Heller A. Pharmacokinetics of high-dose intravenous ciprofloxacin in young and elderly and in male and female subjects. Antimicrob Agents Chemother. 1995. https://doi.org/10.1128/AAC.39.4.1003.
Segev S, Yaniv I, Haverstock D, Reinhart H. Safety of long-term therapy with ciprofloxacin: data analysis of controlled clinical trials and review. Clin Infect Dis. 1999. https://doi.org/10.1086/515132.
Gerding DN, Hitt JA. Tissue penetration of the new quinolones in humans. Rev Infect Dis. 1989. https://doi.org/10.1093/clinids/11.Supplement_5.S1046.
Naber KG, Sörgel F, Kees F, Jaehde U, Schumacher H. Brief report: pharmacokinetics of ciprofloxacin in young (healthy volunteers) and elderly patients, and concentrations in prostatic fluid, seminal fluid, and prostatic adenoma tissue following intravenous administration. Am J Med. 1989. https://doi.org/10.1016/0002-9343(89)90023-5.
Thakur A. Nano therapeutic approaches to combat progression of metastatic prostate cancer. Adv Cancer Biol Metastasis. 2021. https://doi.org/10.1016/j.adcanc.2021.100009.
Acknowledgements
We thank Dr. MR Abbaszadegan and N Taghechian for their kind help with qRT-PCR assay.
Funding
This work was supported by Ferdowsi University of Mashhad, grant number 3/44066. Razieh Jalal has received research support from Ferdowsi University of Mashhad.
Author information
Authors and Affiliations
Contributions
RJ: Designed and conducted the experiments, analyzed the data, and wrote the manuscript. ADA and JG: Performed cell biological experiments and analyzed the data.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Davary Avareshk, A., Jalal, R. & Gholami, J. The effect of ciprofloxacin on doxorubicin cytotoxic activity in the acquired resistance to doxorubicin in DU145 prostate carcinoma cells. Med Oncol 39, 194 (2022). https://doi.org/10.1007/s12032-022-01787-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01787-9