Abstract
Background
Triple-negative breast cancer (TNBC) is one of the most aggressive subtypes of breast cancer. TNBC lacks targeted therapy receptors, rendering endocrine and HER2-targeted therapies ineffective. TNBC is typically treated with cytotoxic chemotherapy followed by surgery. Targeting epigenetic modifications could potentially be a new effective TNBC target therapy. The aim of this study is to examine the effects of epigenetic drugs, decitabine as DNA methyltransferase inhibitor (DNMTI) and vorinostat as histone deacetylase inhibitor (HDACI), and the ERβ agonist DPN on ERα and ERβ re-expressions in the MDA-MB-231 cells as a model of TNBC.
Methods
Using MTT assay, the IC50 of decitabine, vorinostat, and DPN on MDA-MB-231 cells were determined. The effects of all drugs alone or in combinations on MDA-MB-231 cells were evaluated. qRT-PCR was used to determine ERα & ERβ gene expression. Caspase-3 activity and the protein expression levels of VEGF, Cyclin D1, and IGF-1 were assessed.
Results
Both ERα and ERβ mRNA were re-expressed in different high levels in all treated groups, especially in the triple therapy group compared with control. Significantly, the triple drugs therapy showed the lowest levels of VEGF, Cyclin D1, and IGF-1 and the highest level of Caspase-3 activity, indicating a possible antitumor effect of ERβ activation through decreasing proliferation and angiogenesis and increasing apoptosis in MDA-MB-231 cells.
Conclusions
The antiproliferative effect of ERβ could be retained when co-expressed with Erα using a powerful epigenetic combination of Decitabine and vorinostat with DPN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, breast cancer (BC) is the most diagnosed cancer and the leading cause of cancer death in women, with 24.5% incidence and 15.5% mortality according to global cancer statistics 2020 [1]. Triple-negative breast cancer (TNBC) is one of the most aggressive subtypes of BC accounting for 15–20% of all BC cases but is responsible for over 50% of BC mortality. TNBC has limited treatment options due to the lack of expression of three receptors: the estrogen receptor alpha (ERα), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) amplification [2]. Therefore, current hormonal or HER2-targeted therapies are not viable for TNBCs; cytotoxic chemotherapy is still the only standard treatment option available despite harsh side effects [3, 4]. Although TNBC patients have a better clinical response to chemotherapy, they have a worse prognosis than other BC subtypes. [5]. Therefore, there is an urgent need to identify better treatment options that are less toxic and are more targeted to TNBC patients.
Despite the lack of ERα in TNBCs, the discovery of estrogen receptor beta (ERβ) expression in some TNBC subtypes made it a possible logical therapeutic target [6, 7]. Unlike the tumorigenesis effect of ERα, ERβ has been suggested to act as a tumor suppressor in breast tissue because its expression declines during carcinogenesis, its knockdown increases the proliferation of mammary epithelial and BC cells. In contrast, its overexpression inhibited tumor cells proliferation, acting as a brake [8, 9]. In general, ERβ activity is considered antagonistic to that of ERα when both receptors co-expressed together in a cell [10]; thus, activation of ERβ by specific agonists is suggested to be a feasible treatment option for BC, including TNBC [11]. One of the first synthetic ERβ selective agonists reported to have a high affinity for ERβ is DPN (2,3-Bis (4-hydroxyphenyl) propionitrile, diaryl propionitrile), which was used to examine the role of ERβ in different TNBC studies [5].
Epigenetic modifications, such as DNA methylation and histones acetylation, are heritable epigenetic processes that regulate gene expression in normal mammalian development [12]. TNBCs show extensive promoter hypermethylation of critical genes such as tumor suppressors and ERs compared with other BC subtypes; thus, targeting epigenetic regulators showed promising benefits in a series of TNBC cells [13].
It is well-known that the epigenetic silencing of the ERα gene in ER-negative human BCs involves interactions between DNA methyltransferases (DNMTs) and histone deacetylases (HDACs), which associated with DNA hypermethylation and histone hypoacetylation to maintain a stable repressive chromatin complex in the silenced ER promoter [12]. In harmony, studies demonstrated that ERβ expression is regulated by DNA methylation and histone acetylation. Hypermethylation of ESR2 promoter was associated with a marked decrease of ERβ mRNA expression in BCs, while inhibition of DNMTs reactivated ERβ expression. In both ERα -positive luminal and ERα -negative basal-like BC cells, HDAC inhibitors (HDACIs) increased ERβ expression [14]. Therefore, several studies supported using a combination of DNMT inhibitors (DNMTIs) and HDACIs for ERs re-expression in TNBCs [12].
Decitabine (5-aza-2′deoxycytidine) is a DNMTI that is approved by the US-FDA for treating hematological malignancies. Decitabine suppresses all 3 DNMTs: DNMT1, DNMT3A, and DNMT3B causing partial demethylation of the ER CpG island and restores expression of functional ER in ER-negative human BC cells [13]. Vorinostat (Suberanilohydroxamic acid, SAHA) HDACI, is the first in its class to be approved by the US-FDA to treat cutaneous T-cell lymphoma. It inhibits class I and II HDACs, including HDAC1, HDAC2, HDAC3, and HDAC6, at low micromolar concentrations [12].
There is mounting evidence that a combination of HDACI, such as vorinostat, with DNMTI, such as decitabine, can restore ERα expression and sensitize ER-negative BCs like MDA-MB-231 cells to hormone therapy or chemotherapy [12]. Therefore, reactivating both of ERα and ERβ expressions in MDA-MB-231 TNBC cells that are a well-characterized model that does not endogenously express any form of the ERs [6] could restore the antiproliferative effect of ERβ in the presence of ERα using a selective ERβ agonist such as DPN.
In the present study, we examine the antitumor effect of DPN (ERβ agonist) after re-expression of ERα and ERβ using the powerful epigenetic combination of Decitabine (DNMTI) and vorinostat (HDACI) for treatment of MDA-MB-231 cells (TNBC cell line).
Materials and methods
Chemicals
Decitabine and Vorinostat (code: s1200 and s1047, respectively) were purchased from SelleckChem, USA. DPN (code: 1494) was purchased from Tocris Bioscience, (UK). All other chemicals and materials were commercially available and of standard quality.
Experimental cell lines
The MDA-MB-231 cell line was supplied from the American Type Culture Collection (ATTC, Manassas, VA, USA). According to method described previously [15], Cells were cultured in DMEM supplemented with 1% penicillin/streptomycin and 10% FBS and incubated at 37 °C in the presence of 5% CO2 and 95% humidified air. Cells were harvested at 80% confluence using a 2.5% (w/v) trypsin solution and subculture into T-75 flasks or 96-well plates, depending on the experiment.
Cell viability assay
According to the method described previously [15], The MTT assay was used to determine the effects of Decitabine, Vorinostat, or DPN on cell viability. MDA-MB-231 cells were seeded in a 96-well plate at a density of 4000 cells per well, with each well containing 100 µl DMEM medium supplemented with 10% FBS and incubated overnight at 37 °C in 5% CO2, 95% air until 70–80% confluence.
The Old media was aspirated and then 200 μl of DMEM containing different drug concentrations was added to all wells except control wells and incubated for another 72 h.
-
Decitabine concentrations (0.5, 1, 2, 4, 8 and 16 μM) [16].
-
Vorinostat concentrations (0.0187, 0.0375, 0.075, 0.15, 0.3 and 0.6 μM) [17].
-
DPN concentrations (0.005, 0.01, 0.02, 0.04, 0.08 and 0.16 μM) [5].
The media were then aspirated, and cells were incubated in the dark for four hours in the dark with 20 μl MTT working solution (5 mg/ml in DMEM). After removing the supernatant, the resulting purple formazan crystals were dissolved in 150 μl of DMSO over a 15 min period of agitation. Absorbance was recorded at 590 nm using a microplate reader. Each experiment was repeated at least three times independently in triplicate. As mentioned in previous study [18], the cells’ viability was expressed as a percentage relative to control. Median inhibitory concentration (IC50) values were determined using CompuSyn software (CompuSyn, Inc., version 1).
Experimental design and treatment of MDA-MB-231 cells with drugs
At first, equal numbers of cells (2 × 105 cells/flask) [15] were cultured in 24 identical T-25 culture flasks and incubated at 37 °C in 5% CO2. After 48 h of incubation, cell viability and confluency of the flasks were checked to be 70–80%.
The 24T-25 seeded flasks were divided into eight groups each group contained three flasks, treated as follows:
-
Group I (n = 3): control group treated with complete media only as a vehicle.
-
Group II (n = 3): treated with Decitabine (4 μM)
-
Group III (n = 3): treated with Vorinostat (0.26 μM)
-
Group IV (n = 3): treated with DPN (0.093 μM)
-
Group V (n = 3): treated with Decitabine (4 μM) and Vorinostat (0.26 μM)
-
Group VI (n = 3): treated with DPN (0.093 μM) and Vorinostat (0.26 μM)
-
Group VII (n = 3): treated with DPN (0.093 μM) and Decitabine (4 μM)
-
Group VIII (n = 3): treated with DPN (0.093 μM), Vorinostat (0.26 μM) and Decitabine (4 μM)
All treatments were applied at 70–80% confluence, and the cells were incubated in a CO2 incubator for 72 h; then, the cells were harvested and portioned into aliquots. The total protein content of each aliquot was quantified via the method reported by Bradford MM, 1976 [19]. Finally, the aliquots were kept at − 80 °C for further investigations.
Preparation of cell lysates
Cell lysates were prepared using RIPA lysis and extraction buffer, purchased from Thermo Scientific, USA (Catalog Number: 89900). As directed by the manufacturer, 1 ml of cold RIPA buffer was added to 40 mg of wet cell pellets, which were then kept on ice and gently shaken for 15 min and then centrifuged at 14,000×g for 15 min to pellet the cell debris. After that, the supernatants were transferred to new tubes and stored at 20 °C for subsequent analysis.
Biomarker analysis using ELISA technique
Vascular endothelial growth factor (VEGF), insulin-like growth factor 1 (IGF-1), and cyclin D1 were evaluated in the cell lysates from different treatment groups using the ELISA technique using Human VEGF ELISA kit (Cusabio Biotech Co., LTD, China, code: CSB-E11718h), and Human IGF-1 ELISA kit (Abnova, USA, code: KA0349), and Human Cyclin-D1 ELISA kit (Eiaab Science Inc, Wuhan, China, code: E0585h).
The manufacturer's protocol was followed in all measurements. Each parameter was assayed in triplicate and was expressed relative to the total protein content in the same sample.
Caspase‑3 activity assay
Caspase-3 activity was measured using a colorimetric kit (Caspase-3 Assay Kit, Colorimetric (Sigma-Aldrich, USA, code: CASP-3-C), according to the manufacturer's procedure.
Quantitative real-time polymerase chain reaction (qRT‑PCR)
According to the manufacturer’s protocols, A total RNA extraction kit, easy-RED™ Total RNA Extraction Kit (iNtRON Biotechnology, S.Korea, Catalog Number: 17063), was used to extract total RNA, then The RNA was reverse transcribed using a TOPscript™ cDNA Synthesis kit (Enzynomics, S.Korea, Catalog Number: EZ005S). Using qRT-PCR (DTlite Real-Time PCR system), ERα and ERβ gene expression was determined by a TOPreal™ qPCR 2X PreMIX (SYBR Green with low ROX) (Enzynomics, S.Korea, Catalog Number: RT500S) using a housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primer pair sequences are shown in Table 1. The assessment of each specimen was carried out in triplicate, and the fold changes in ERα and ERβ gene expression were calculated as described by Livak et al. [20].
Statistical analysis
The data were expressed as the mean ± standard error of the mean (SEM). Multiple comparisons were analyzed using a one-way analysis of variance (ANOVA) followed by a post hoc Tukey’s multiple comparison test., and the differences were considered significant at p < 0.05. GraphPad Prism® software package version 8.0.2 (GraphPad Software Inc., CA, USA) was used for all statistical analyses and data presentation.
Results
IC50 values of decitabine, vorinostat, and DPN in MDA-MB-231 cell line
Effects of decitabine, vorinostat, and DPN on MDA-MB-231 cells viability are shown in Fig. 1, respectively. Drugs showed concentration-dependent cytotoxic effects, where treating cells with decitabine concentrations (0.50, 1, 2, 4, 8, and 16 μM) inhibited the cell viability with an IC50 of 4 μM. Similarly, vorinostat treatment at different concentrations (0.0187, 0.0375, 0.075, 0.15, 0.30 and 0.60 μM) potently inhibited cell growth with an IC50 of 0.26 μM, and DPN at concentrations (0.005, 0.01, 0.02, 0.04, 0.08 and 0.16 μM) inhibited cell growth with an IC50 of 0.093 μM.
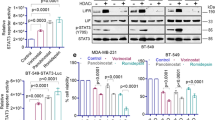
Effects of decitabine, vorinostat, DPN and their combinations on ERα and ERβ genes expression in MDA-MB-231 cells
Figure 2 illustrates that incubation of MDA-MB-231 cells with decitabine, vorinostat, or DPN alone induced both ERα and ERβ mRNA expression at different levels in groups (II-IV) compared to the control group (I). Using a combination of decitabine and vorinostat as epigenetic drugs in the group (V) showed high induction of both ERα and ERβ genes expression with an obvious greater increase of ERα expression level by 220.9 and 6.4 folds compared to using decitabine or vorinostat alone, respectively. The addition of DPN (ERβ agonist) upregulated ERβ expression in all its combinations groups (VI-VIII) especially the triple combination therapy group (VIII), which showed the highest ERβ expression level of all treatment groups compared to the vehicle control group. On the other hand, DPN downregulated ERα expression in the triple therapy group (VIII) by 2.8-fold in comparison with its highest expression in the double epigenetic drugs combination (decitabine and vorinostat) in the group (V).
ERβ expression and/or activation reduced cyclin D1 and IGF-1 protein levels when co-expressed with ERα in MDA-MB-231 cells
Figure 3A and B shows that decitabine, vorinostat, DPN, and their combinations affected the expression of both proliferation markers cyclin D1 and IGF-1 with different extents. Both cyclin D1 and IGF-1 proteins levels were significantly reduced (p < 0.001) in all treated cells groups (II–VIII) compared to their highest levels in the untreated control group (I).
Effects of decitabine (4 µM), vorinostat (0.26 µM), DPN (0.093 µM) and their combinations treatments on the treated groups of MDA-MB-231 cells A cyclin D1, B IGF-1 C active caspase-3 activity, and D VEGF biomarkers. Data are presented as the mean ± SEM of three samples each performed in triplicate. Statistically significant differences between groups (p < 0.05) are designated as *significant vs. control, πsignificant vs. DPN, #significant vs. decitabine, $significant vs. vorinostat, Δsignificant vs. decitabine & vorinostat
It was noticed that cells treated with combination therapies groups (V–VIII) remarkably reduced both cyclin D1 and IGF-1 proteins levels more than using each drug alone, and the greatest reduction of cyclin D1 by 12.2-fold and of IGF-1 by11.6-fold compared to the control group was observed in cells that displayed the highest level of ERβ expression and activation, the triple therapy group (VIII). Hence, the data raised the idea that ERβ enhanced expression and/or activation can exert an antiproliferative effect when co-expressed with ERα in MDA-MB-231 cells.
ERβ expression and/or activation stimulated apoptosis and enhanced caspase‑3 activity when co-expressed with ERα in MDA-MB-231 cells
To investigate the effect of decitabine, vorinostat, DPN and their combinations along with ERβ expression and/or activation on apoptosis, the caspase-3 activity was assessed as an apoptotic marker. Figure 3C shows that the caspase-3 activity significantly increased (p < 0.001) in all treated groups (II–VIII) in comparison with the lowest level observed in the control group (I). In addition, the caspase-3 activity in cells receiving combined treatments groups (V–VIII) was significantly higher (p < 0.001) than treated cells receiving single-agent treatments, specially DPN drug combinations.
Of interest, the highest caspase-3 activity was detected in cells treated with triple therapy, which exhibited the highest level of ERβ expression and activation, indicating the greatest apoptotic effect. These results suggested that ERβ expression and/or activation contributed to the induction of apoptosis in the presence of ERα expression in MDA-MB-231 cells.
ERβ expression and/or activation influences angiogenesis by reducing VEGF protein levels when co-expressed with ERα in MDA-MB-231 cells
Figure 3D shows the effects of decitabine, vorinostat, DPN and their combinations on the angiogenesis marker VEGF protein level in MDA-MB-231 treated cells. VEGF protein levels in all treated groups (II–VIII) were significantly decreased (p < 0.001) compared to the highest level in the control group (I). Additionally, cells exposed to the combined treatments groups (V–VIII) showed a significant decrease over the single treatment action in groups (II–VI) compared to the control group. Interestingly, the lowest level of VEGF and so angiogenesis was observed in cells treated with the three-drug combination, which had the highest level of ERβ expression and activation.
Discussion
Because of the lack of target receptors, TNBC patients do not benefit from hormonal or HER2-targeted therapies. TNBC tumors showed extensive promoter hypermethylation of epigenetic biomarker genes compared with other BC subtypes, it was reported that both ERs’ expression is epigenetically controlled [21].
However, there have been conflicting results concerning the function and clinical value of ERβ, especially in TNBC. Several studies suggested that ERβ positivity is of a favorable prognostic value for TNBC [7, 22], no prognostic value [23, 24], or worse prognosis and correlates with aggressive phenotypes [5, 25]. Potential reasons for these discrepancies can be since TNBC is a heterogeneous disease of different subtypes [5]; others suggested that due to the existence of at least five different ERβ receptor isoforms (ERβ1-5) in human BCs whose biological functions largely remain controversial [6]. Despite these discrepancies, several studies demonstrated the antitumor effect of ERβ activation using selective agonists in ERβ positive TNBCs, suggesting that endocrine therapy options targeting ERβ should be considered to treat patients with TNBC [11, 26]. Therefore, targeting ERβ after its re-expression using epigenetic drugs could be a promising therapeutic strategy of ERβ negative TNBCs treatment.
In the present study, we examined the effects of epigenetic drugs, decitabine as DNMTI and vorinostat as HDACI, and the ERβ agonist DPN on ERα and ERβ expressions in the MDA-MB-231 cells which is a well-characterized model of TNBC that do not endogenously express any form of the estrogen receptor neither ERβ nor ERα [6].
Our results showed that using each epigenetic drug alone (decitabine or vorinostat) caused re-expression of both ERα and ERβ mRNA at different levels with a higher effect of vorinostat than decitabine while using both decitabine and vorinostat as an epigenetic combination treatment showed a high re-expression effect of both receptors with a substantially greater increase of ERα expression than each drug alone as expected. That agreed with mounting studies which demonstrated the ability of HDACIs, such as vorinostat, to reactivate ERα expression at both transcriptional and protein levels in ER-negative BC cell lines like MDA-MB-231 and in different aggressive subtypes of TNBC through an epigenetic mechanism, and they sensitized cells to the anti-estrogen drug tamoxifen [12, 27]. Additionally, studies reported that HDACIs like trichostatin A induced re-expression of ERβ in BC cell lines, including TNBC cells, that was also observed in the ovary and prostate cancer cell lines [14, 28, 29]. In several studies, decitabine treatment was associated with an increased re-expression of ERβ in BC [30] and prostate cancer cells [14].
The observed greater effect of the epigenetic drugs combination compared to each drug alone corroborates with other studies which showed that the combination of DNMTIs and HDACIs demonstrated a synergistic effect on reactivation of silenced genes in cancer besides their beneficial anti-tumor effects [14, 29, 31], as shown in MDA-MB-231 and other TNBC cell lines through re-expression of ERs after application of this combination [12, 32].
In early clinical trials, decitabine did not show a major therapeutic effect when administered as monotherapy, however, preclinical and clinical studies of different cancer entities showed evidence of a synergistic effect of decitabine in combination with HDACIs to restore ERα expression and sensitize ER-negative BCs to hormone therapy or chemotherapy [33, 34]. Studies demonstrated that decitabine and trichostatin A co-administration potentiated the re-expression of ERβ in breast, ovary, and prostate cancer cell lines, and induced apoptosis, cell differentiation, and growth termination [14, 35]. Confirming that it was the best choice for our study to use this epigenetic combination (decitabine and vorinostat) together better than using each drug alone to re-express both ERs.
Additionally, DPN alone demonstrated an upregulation effect on ERβ expression. Similarly, several studies indicated that DPN induced ERβ expression in prostate cancer cells, which may be attributed to the receptor autoregulation caused by the presence of ERE sequences in the distant promoter region of the human ERβ gene [14, 36, 37]. This finding evoked hopes that ERβ agonists could be used clinically to upregulate ERβ expression in the early stages of cancer and thus prevent proliferation and progression [38].
The addition of DPN had a marked upregulation effect on ERβ expression in all of its combination groups (VI-VIII), especially the triple combination therapy group (VIII), which showed the highest ERβ expression level (56.04-fold) compared to the vehicle control group as well as to other treated groups. These data suggested an augmented effect between epigenetic treatment and receptor activation on ERβ expression in MDA-MB-231 cells. On the other hand, DPN as an ERβ agonist showed a negative effect and markedly decreased ERα re-expression levels, which appeared especially in the triple therapy group (VIII) compared to the highest expression level of ERα in (decitabine + vorinostat) epigenetic combination treatment group (V). That was consistent with several previous studies which indicated that ERβ downregulates ERα expression when co-expressed together via heterodimerization with ERα and increased ERα proteolytic degradation; as a result, ERβ can inhibit ERα activity and its proliferation effect [29, 39]. The antitumor effects of ERβ expression and activation were investigated and confirmed by measuring the other parameters.
Concerning the anti-proliferative effect of ERβ, the exact role of ERβ in BC is controversial; both proliferative and anti-proliferative ERβ roles have been described [40]. Our study aimed to examine the anti-proliferation effect of ERβ expression and activation when co-expressed with ERα, so we determined both cyclin D1 and IGF-1 protein levels in MDA-MB-231 cells as proliferation biomarkers.
Cyclin D1 is a cell cycle-related protein responsible for the transition from the (G1) phase to the (S) phase in the cell cycle. Its overexpression has been described in several human malignancies, including BC [14]. Alterations in IGF-1/IGF-1R signaling mediate stimulatory effects in malignant cells. High IGF-1R expression and elevated IGF-1 circulating levels have been correlated with the increased risk and progression of BC with poor prognosis through promoting cell proliferation, invasion, anti-apoptosis, and tumor angiogenesis [41, 42]. In particular, it had been shown that approximately 30–40% of TNBCs harbors amplification of the IGF-1R gene, which was linked to a short survival rate of these patients [43]. Additionally, high IGF-1 gene expression or IGF-1R (or both) levels were correlated with a worse clinical outcome in TNBC patients and triggered the growth potential, proliferation, and invasion of TNBC, including MDA-MB-231 contributing to the progression of more aggressive TNBC subtypes with poor survival [43,44,45].
Our results showed that re-expression of ERβ significantly reduced protein levels of both cyclin D1 and IGF-1 in all treated groups compared to the control group indicating the antiproliferative effect of ERβ expression. That was consistent with various studies that demonstrated the anti-proliferative effect of ERβ in MDA-MB-231 and MDA-MB-468 TNBC cell lines after ERβ exogenous expression and its activation by E2 or specific agonists such as DPN, which was able to notably inhibit TNBC cell growth, arrest cell cycle at the G1 phase, block cell colony formation, inhibit cell invasiveness and reduce tumor size in mice xenografts [11, 46]. A recent study confirmed that overexpression of ERβ using adenoviral infection as a means to elevate ERβ levels was found to suppress the proliferation, migration, and invasion of the MDA-MB-231 cells and in other TNBC subtypes [47] consistent with other studies which found that ERβ had a tumor-suppressive effect [11, 48].
In contrast to our results, some studies reported that ERβ overexpression increased the rate of cell proliferation and progression in some TNBC subtypes; in addition, ERβ positivity in TNBC was correlated with higher expression of the proliferation marker Ki- 67 [5]. In another study, targeting ERβ with DPN in T47D cells (ERα- positive/ERβ1-positive) had a little to no effect on the proliferation rates [6, 49].
Interestingly, our results indicated that the re-expression of ERβ and ERα together in high levels using the epigenetic drugs combination group (V) displayed more reduction of cyclin D1 and IGF-1 protein levels than decitabine, vorinostat, or DPN alone (groups II, III, and IV, respectively). This antiproliferative effect was markedly enhanced through ERβ activation using its agonist DPN combined with the epigenetic drugs, especially in the triple therapy group (VIII), which displayed the highest ERβ expression and so the highest antiproliferation effect. Consequently, these results proved that increasing ERβ expression level using the epigenetic drugs combined with its activation had a crucial role in obtaining a stronger ERβ antiproliferative effect and better than using ERβ agonist alone.
It was important to note that co-expression of both ERs together had a substantial effect on ERβ actions. In contrast to the confused roles of ERβ in TNBC when expressed alone, data suggested that the biological effects of ERβ are critically correlated with the presence of ERα [5, 6]. ERβ appeared to oppose ERα actions on cell proliferation by modulating the expression of many ERα regulated genes [50]. When both ERs co-expressed in cells, ERβ can antagonize ERα-dependent transcription and inhibit ERα proliferative role via alteration of key transcription factors recruitment, heterodimerization and increase ERα proteolytic degradation [29, 39]. In this context, studies showed that ERα- positive/ERβ1-positive tumors typically had reduced expression of Ki67 relative to ERα-positive/ERβ1-negative tumors confirming the tumor suppressor role of ERβ in the ERα-positive BC cell lines; additionally, ERβ expression diminished the pro-proliferative effects of ERα and exerted its oncosuppressive role targeting cell division [51, 52].
Gene expression analysis reported that gene expression of cyclin D1 is regulated by estrogen via AP-1 site, which is stimulated by ERα, while is inhibited by ERβ, suggesting that ERβ may modulate the proliferative effects of ERα by blocking its action on the cyclin D1 gene [53]. In tune, a study of a human cervical cancer cell line (Hela cells) had previously demonstrated that E2-activated ERβ acted as a negative regulator of cyclin D1 gene transcription and effectively abrogated the ER-α-mediated activation of cyclin D1 expression when both ER subtypes are co-expressed [54].
These reports collectively were consistent with our finding that targeting the highest level of ERβ with DPN, after its co-expression with ERα using the epigenetic drugs in the triple therapy group, was a crucial step that resulted in obtaining the greatest antiproliferative effect among all treated groups.
Concerning the effect of ERβ re-expression and activation in the presence of ERα on apoptosis, treatment with either decitabine, vorinostat, or DPN alone significantly enhanced caspase-3 activity which is a member of the proteases family that mediate cell death and is one of the critical enzymes of the apoptosis process [55]. This observation supported the belief that ERβ exerts apoptotic effects on various malignant cells [56, 57]. The majority of data from the research on clinical samples and cell lines suggested that ERβ has antiproliferative, tumor-suppressive functions and induces apoptosis in ERα low or negative BC cell lines [29, 58]. Exogenous expression of ERβ was reported to exert apoptotic effects in prostate carcinoma cells [59].
This work showed that cells exposed to combination therapies in groups (V–VIII) exhibited significantly higher levels of caspase-3 activity compared to the single agents applied alone and to the vehicle control group. In tune, studies demonstrated that co-treatment of prostate cancer cells with (decitabine + trichostatin A) was associated with a significant increase in apoptotic activity compared with the single agents alone [14], an effect that seemed to be related to increased ERβ expression. Remarkably, in the present study, the highest level of ERβ expression and activation (in the presence of ERα) in the three-drug combination group was accompanied by the highest level of caspase-3 activity and so apoptosis. The same results were observed after induction of ERβ expression in a prostate cancer cell line in a study that followed the same idea of our work about using a triple therapy of epigenetic drugs and ERβ agonist (decitabine + trichostatin A + DPN) [14]. This observation suggested that triple therapy has the greatest tendency to induce apoptosis via activation of the highest level re-expressed ERβ in the presence of ERα in MDA-MB-231 cells.
Angiogenesis was also estimated by measuring VEGF, which is a key regulator of angiogenesis and can stimulate endothelial cell proliferation to form new blood vessels that support tumor growth and increase the risk of tumor invasion, metastasis, and patient mortality [60]. VEGF overexpression has been described in solid malignancies, including BC [61, 62]. Previous studies have shown that TNBC possesses high microvessel density and VEGF amplification than non-TNBC [62, 63]. Herein, individual administration of decitabine, vorinostat, or DPN alone caused a significant reduction in VEGF protein level as compared to the control group, but cells exposed to the combined treatments showed a significant decrease over the single-agent action. That was attributed to the increased levels of ERβ expression and activation in the presence of ERα, in addition to the antitumor effect of the epigenetic drugs.
That was in agreement with data that revealed that the specific HDACI, entinostat, attenuated tumor progression and metastasis in TNBC through downregulation of VEGF expression and enhancing the re-expression of anti-angiogenic and tumor suppressor genes epigenetically [62], supporting the antiangiogenic effect of our epigenetic drugs.
In addition, estrogens had been implicated in controlling VEGF expression in target tissues and corresponding tumors through ERα and ERβ [64]. Liu et al. showed that liquiritigenin, an ERβ agonist, reduced tumor growth of HeLa cells in nude mice via inhibition of VEGF expression and so angiogenesis [65]. Similarly, Motawi et al. have demonstrated that re-expression of ERβ followed by activation using DPN treatment attenuated VEGF protein level in the PC-3 prostate cancer cells [14]. Interestingly, in our study, the lowest levels of VEGF and so angiogenesis was observed in cells treated with the three-drug combination, which had the highest levels of ERβ re-expression, supporting that ERβ re-expression and activation are responsible, at least in part, for the downregulation of VEGF protein expression that would eventually repress angiogenesis in MDA-MB-231 cells.
Conclusion
Taken all together, according to the aforementioned evidence, the combinatorial therapy of decitabine, vorinostat, and DPN implied retaining the anti-tumor effect of ERβ as a result of induced ERβ overexpression and activation in the presence of ERα in MDA-MB231 TNBC cells. That may hold promises for those patients with extremely poor outcomes and for which no form of targeted cancer therapy is currently available. Therefore, we recommend further preclinical and clinical studies on different subtypes of TNBC cells to verify the validity of such a promising triple combination. The same notion should be further elevated in other hormone-dependent cancers like prostate, endometrial, and cervical cancer.
Abbreviations
- BC:
-
Breast cancer
- DMEM:
-
Dulbecco's modified eagle's medium
- DMSO:
-
Dimethyl sulfoxide
- DNMTI:
-
DNA methyltransferase inhibitor
- DNMTs:
-
DNA methyltransferases
- DPN:
-
(2,3-Bis (4-hydroxyphenyl) propionitrile)
- Erα:
-
Estrogen receptor alpha
- Erβ:
-
Estrogen receptor beta
- FBS:
-
Fetal bovine serum
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- HDACI:
-
Vorinostat as histone deacetylase inhibitor
- HDACs:
-
Histone deacetylases
- HER2:
-
Human epidermal growth factor receptor 2
- IC50:
-
Median inhibitory concentration
- IGF-1:
-
Insulin-like growth factor 1
- MTT:
-
3-(4, 5-Dimethyl thiazolyl-2)-2, 5-diphenyltetrazolium bromide
- PBS:
-
Phosphate buffer saline
- PR:
-
Progesterone receptor
- qRT‑PCR:
-
Quantitative real‑time polymerase chain reaction
- TNBC:
-
Triple-negative breast cancer
- VEGF:
-
Vascular endothelial growth factor
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49.
Cetin I, Topcul M. Triple negative breast cancer. Asian Pac J Cancer Prev. 2014;15(6):2427–31.
Losurdo A, De Sanctis R, Fernandes B, Torrisi R, Masci G, Agostinetto E, et al. Insights for the application of TILs and AR in the treatment of TNBC in routine clinical practice. Sci Rep. 2020. https://doi.org/10.1038/s41598-020-77043-9.
Network NCC. NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2021;4:2021.
Austin D, Hamilton N, Elshimali Y, Pietras R, Wu Y, Vadgama J. Estrogen receptor-beta is a potential target for triple negative breast cancer treatment. Oncotarget. 2018;9(74):33912.
Reese JM, Suman VJ, Subramaniam M, Wu X, Negron V, Gingery A, et al. ERβ1: Characterization, prognosis, and evaluation of treatment strategies in ERα-positive and-negative breast cancer. BMC Cancer. 2014;14(1):1–16.
Wang J, Zhang C, Chen K, Tang H, Tang J, Song C, et al. ERβ1 inversely correlates with PTEN/PI3K/AKT pathway and predicts a favorable prognosis in triple-negative breast cancer. Breast Cancer Res Treat. 2015;152(2):255–69.
Bado I, Nikolos F, Rajapaksa G, Wu W, Castaneda J, Krishnamurthy S, et al. Somatic loss of estrogen receptor beta and p53 synergize to induce breast tumorigenesis. Breast Cancer Res. 2017;19(1):1–10.
Hawse JR, Carter JM, Aspros KG, Bruinsma ES, Koepplin JW, Negron V, et al. Optimized immunohistochemical detection of estrogen receptor beta using two validated monoclonal antibodies confirms its expression in normal and malignant breast tissues. Breast Cancer Res Treat. 2020;179(1):241–9.
Hamilton N, Márquez-Garbán D, Mah V, Fernando G, Elshimali Y, Garbán H, et al. Biologic roles of estrogen receptor-β and insulin-like growth factor-2 in triple-negative breast cancer. BioMed res int. 2015. https://doi.org/10.1155/2015/925703.
Schüler-Toprak S, Häring J, Inwald EC, Moehle C, Ortmann O, Treeck O. Agonists and knockdown of estrogen receptor β differentially affect invasion of triple-negative breast cancer cells in vitro. BMC Cancer. 2016;16(1):951.
Stark K, Burger A, Wu J, Shelton P, Polin L, Li J. Reactivation of estrogen receptor α by vorinostat sensitizes mesenchymal-like triple-negative breast cancer to aminoflavone, a ligand of the aryl hydrocarbon receptor. PLoS ONE. 2013. https://doi.org/10.1371/journal.pone.0074525.
Yu J, Qin B, Moyer AM, Nowsheen S, Liu T, Qin S, et al. DNA methyltransferase expression in triple-negative breast cancer predicts sensitivity to decitabine. J Clin Investig. 2018. https://doi.org/10.1172/JCI97924.
Motawi TK, Darwish HA, Diab I, Helmy MW, Noureldin MH. Combinatorial strategy of epigenetic and hormonal therapies: a novel promising approach for treating advanced prostate cancer. Life Sci. 2018;198:71–8.
Abd-Alhaseeb MM, Massoud SM, Elsayed F, Omran GA, Salahuddin A. Evening primrose oil enhances tamoxifen’s anticancer activity against breast cancer cells by inducing apoptosis, inhibiting angiogenesis, and arresting the cell cycle. Molecules. 2022;27(8):2391.
Kastl L, Brown I, Schofield AC. Effects of decitabine on the expression of selected endogenous control genes in human breast cancer cells. Mol Cell Probes. 2010;24(2):87–92.
Uehara N, Kanematsu S, Miki H, Yoshizawa K, Tsubura A. Requirement of p38 MAPK for a cell-death pathway triggered by vorinostat in MDA-MB-231 human breast cancer cells. Cancer Lett. 2012;315(2):112–21.
El-Hanboshy SM, Helmy MW, Abd-Alhaseeb MM. Catalpol synergistically potentiates the anti-tumour effects of regorafenib against hepatocellular carcinoma via dual inhibition of PI3K/Akt/mTOR/NF-κB and VEGF/VEGFR2 signaling pathways. Mol Biol Rep. 2021;48(11):7233–42.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–54.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25(4):402–8.
Shin E, Lee Y, Koo JS. Differential expression of the epigenetic methylation-related protein DNMT1 by breast cancer molecular subtype and stromal histology. J Trans Med. 2016. https://doi.org/10.1186/s12967-016-0840-x.
Yan Y, Li X, Blanchard A, Bramwell VHC, Pritchard KI, Tu D, et al. Expression of both estrogen receptor-beta 1 (ER-β1) and its co-regulator steroid receptor RNA activator protein (SRAP) are predictive for benefit from tamoxifen therapy in patients with estrogen receptor-alpha (ER-α)-negative early breast cancer (EBC). Ann Oncol. 2013;24(8):1986–93. https://doi.org/10.1093/annonc/mdt132.
Baek J-M, Chae B-J, Song B-J, Jung S-S. The potential role of estrogen receptor β2 in breast cancer. Int J Surg. 2015;14:17–22.
Wimberly H, Han G, Pinnaduwage D, Murphy LC, Yang XR, Andrulis IL, et al. ERβ splice variant expression in four large cohorts of human breast cancer patient tumors. Breast Cancer Res Treat. 2014;146(3):657–67.
Guo L, Meng J, Yilamu D, Jakulin A, Fu M, Wang B, et al. Significance of ERβ expression in different molecular subtypes of breast cancer. Diagn Pathol. 2014;9(1):1–6.
Reese JM, Bruinsma ES, Monroe DG, Negron V, Suman VJ, Ingle JN, et al. ERβ inhibits cyclin dependent kinases 1 and 7 in triple negative breast cancer. Oncotarget. 2017;8(57):96506.
Nouriemamzaden F, Word B, Cotton E, Hawkins A, Littlejohn K, Moore R, et al. Modulation of estrogen α and progesterone receptors in triple negative breast cancer cell lines: the effects of vorinostat and indole-3-carbinol in vitro. Anticancer Res. 2020;40(7):3669–83.
Duong V, Licznar A, Margueron R, Boulle N, Busson M, Lacroix M, et al. ER α and ER β expression and transcriptional activity are differentially regulated by HDAC inhibitors. Oncogene. 2006;25(12):1799–806.
Božović A, Mandušić V, Todorović L, Krajnović M. Estrogen receptor beta: the promising biomarker and potential target in metastases. Int J Mol Sci. 2021;22(4):1656.
Rody A, Holtrich U, Solbach C, Kourtis K, von Minckwitz G, Engels K, et al. Methylation of estrogen receptor β promoter correlates with loss of ER-β expression in mammary carcinoma and is an early indication marker in premalignant lesions. Endocr Relat Cancer. 2005;12(4):903–16. https://doi.org/10.1677/erc.1.01088.
Cameron EE, Bachman KE, Myöhänen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21(1):103–7.
Fan J, Yin W-J, Lu J-S, Wang L, Wu J, Wu F-Y, et al. ERα negative breast cancer cells restore response to endocrine therapy by combination treatment with both HDAC inhibitor and DNMT inhibitor. J Cancer Res Clin Oncol. 2008;134(8):883–90.
Yu J, Zayas J, Qin B, Wang L. Targeting DNA methylation for treating triple-negative breast cancer. Pharmacogenomics. 2019;20(16):1151–7.
Schröder R, Illert A-L, Erbes T, Flotho C, Lübbert M, Duque-Afonso J. The epigenetics of breast cancer–opportunities for diagnostics, risk stratification and therapy. Epigenetics. 2021. https://doi.org/10.1080/15592294.2021.1940644.
Vrtačnik P, Ostanek B, Mencej-Bedrač S, Marc J. The many faces of estrogen signaling. Biochemia medica. 2014;24(3):329–42.
Pravettoni A, Mornati O, Martini P, Marino M, Colciago A, Celotti F, et al. Estrogen receptor beta (ERbeta) and inhibition of prostate cancer cell proliferation: studies on the possible mechanism of action in DU145 cells. Mol Cell Endocrinol. 2007;263(1–2):46–54.
Li L-C, Yeh C-C, Nojima D, Dahiya R. Cloning and characterization of human estrogen receptor β promoter. Biochem Biophys Res Commun. 2000;275(2):682–9.
Warner M, Huang B, Gustafsson J-A. Estrogen receptor β as a pharmaceutical target. Trends Pharmacol Sci. 2017;38(1):92–9.
Coriano CG, Liu F, Sievers CK, Liang M, Wang Y, Lim Y, et al. A computational-based approach to identify estrogen receptor α/β heterodimer selective ligands. Mol Pharmacol. 2018;93(3):197–207.
Lattrich C, Stegerer A, Häring J, Schüler S, Ortmann O, Treeck O. Estrogen receptor β agonists affect growth and gene expression of human breast cancer cell lines. Steroids. 2013;78(2):195–202.
Hormones TE, Group BCC. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11(6):530–42.
De Francesco EM, Sims AH, Maggiolini M, Sotgia F, Lisanti MP, Clarke RB. GPER mediates the angiocrine actions induced by IGF1 through the HIF-1α/VEGF pathway in the breast tumor microenvironment. Breast Cancer Res. 2017;19(1):1–14.
Rigiracciolo DC, Nohata N, Lappano R, Cirillo F, Talia M, Scordamaglia D, et al. IGF-1/IGF-1R/FAK/YAP transduction signaling prompts growth effects in triple-negative breast cancer (TNBC) cells. Cells. 2020;9(4):1010.
Daubriac J, Han S, Grahovac J, Smith E, Hosein A, Buchanan M, et al. The crosstalk between breast carcinoma-associated fibroblasts and cancer cells promotes RhoA-dependent invasion via IGF-1 and PAI-1. Oncotarget. 2018;9(12):10375.
Zou Y, Zheng S, Xiao W, Xie X, Yang A, Gao G, et al. circRAD18 sponges miR-208a/3164 to promote triple-negative breast cancer progression through regulating IGF1 and FGF2 expression. Carcinogenesis. 2019;40(12):1469–79.
Treeck O, Schüler-Toprak S, Ortmann O. Estrogen actions in triple-negative breast cancer. Cells. 2020;9(11):2358.
Yan S, Dey P, Ziegler Y, Jiao X, Kim SH, Katzenellenbogen JA, et al. Contrasting activities of estrogen receptor beta isoforms in triple negative breast cancer. Breast Cancer Res Treat. 2021;185(2):281–92.
Reese JM, Bruinsma ES, Nelson AW, Chernukhin I, Carroll JS, Li Y, et al. ERβ-mediated induction of cystatins results in suppression of TGFβ signaling and inhibition of triple-negative breast cancer metastasis. Proc Natl Acad Sci. 2018;115(41):E9580–9.
Lattrich C, Schüler S, Häring J, Skrzypczak M, Ortmann O, Treeck O. Effects of a combined treatment with tamoxifen and estrogen receptor β agonists on human breast cancer cell lines. Arch Gynecol Obstet. 2014;289(1):163–71.
Gustafsson J-A, Strom A, Warner M. Update on ERbeta. J Steroid Biochem Mol Biol. 2019;191: 105312.
Omoto Y, Iwase H. Clinical significance of estrogen receptor β in breast and prostate cancer from biological aspects. Cancer Sci. 2015;106(4):337–43.
Haldosén L-A, Zhao C, Dahlman-Wright K. Estrogen receptor beta in breast cancer. Mol Cell Endocrinol. 2014;382(1):665–72.
Zilli M, Grassadonia A, Tinari N, Di Giacobbe A, Gildetti S, Giampietro J, et al. Molecular mechanisms of endocrine resistance and their implication in the therapy of breast cancer. Biochimica et Biophysica Acta (BBA)-Rev Cancer. 2009;1795(1):62–81.
Liu M-M, Albanese C, Anderson CM, Hilty K, Webb P, Uht RM, et al. Opposing action of estrogen receptors α and β on cyclin D1 gene expression. J Biol Chem. 2002;277(27):24353–60.
Khalilzadeh B, Shadjou N, Kanberoglu GS, Afsharan H, De La Guardia M, Charoudeh HN, et al. Advances in nanomaterial based optical biosensing and bioimaging of apoptosis via caspase-3 activity: a review. Microchim Acta. 2018;185(9):1–19.
Gustafsson J-A, Ström A. Antiproliferative and pro-apoptotic actions of oestrogen receptor β in prostate cancer. Hamdan Med J. 2014;7(3):403.
Yu C-P, Ho J-Y, Huang Y-T, Cha T-L, Sun G-H, Yu D-S, et al. Estrogen inhibits renal cell carcinoma cell progression through estrogen receptor-β activation. PLoS ONE. 2013;8(2): e56667.
Anestis A, Sarantis P, Theocharis S, Zoi I, Tryfonopoulos D, Korogiannos A, et al. Estrogen receptor beta increases sensitivity to enzalutamide in androgen receptor-positive triple-negative breast cancer. J Cancer Res Clin Oncol. 2019;145(5):1221–33.
Cheng J, Lee EJ, Madison LD, Lazennec G. Expression of estrogen receptor β in prostate carcinoma cells inhibits invasion and proliferation and triggers apoptosis. FEBS Lett. 2004;566(1–3):169–72.
Wang XW, Yu C-P, Ho J-Y, Huang Y-T, Cha T-L, Sun G-H, et al. Estrogen inhibits renal cell carcinoma cell progression through estrogen receptor-β activation. PLoS ONE. 2013;8(2): e56667. https://doi.org/10.1371/journal.pone.0056667.
Roberts E, Cossigny DA, Quan GM. The role of vascular endothelial growth factor in metastatic prostate cancer to the skeleton. Prostate Cancer. 2013. https://doi.org/10.1155/2013/418340.
Maiti A, Qi Q, Peng X, Yan L, Takabe K, Hait N. Class I histone deacetylase inhibitor suppresses vasculogenic mimicry by enhancing the expression of tumor suppressor and anti-angiogenesis genes in aggressive human TNBC cells. Int J Oncol. 2019. https://doi.org/10.3892/ijo.2019.4796.
Mohammed RA, Ellis IO, Mahmmod AM, Hawkes EC, Green AR, Rakha EA, et al. Lymphatic and blood vessels in basal and triple-negative breast cancers: characteristics and prognostic significance. Mod Pathol. 2011;24(6):774–85.
Hyder SM, Nawaz Z, Chiappetta C, Stancel GM. Identification of functional estrogen response elements in the gene coding for the potent angiogenic factor vascular endothelial growth factor. Can Res. 2000;60(12):3183–90.
Liu Y, Xie S, Wang Y, Luo K, Wang Y, Cai Y. Liquiritigenin inhibits tumor growth and vascularization in a mouse model of HeLa cells. Molecules. 2012;17(6):7206–16.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research received no external funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by HG, MWH and AS. The first draft of the manuscript was written by HG, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
This study was approved by the ethical committee of the Faculty of Pharmacy, Damanhour University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salahuddin, A., Ghanem, H., Omran, G.A. et al. Epigenetic restoration and activation of ERβ: an inspiring approach for treatment of triple-negative breast cancer. Med Oncol 39, 150 (2022). https://doi.org/10.1007/s12032-022-01765-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01765-1