Abstract
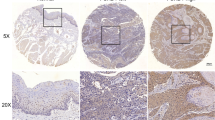
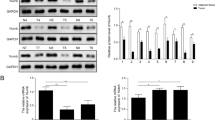
Oral squamous cell carcinoma (named OSCC) is considered the most frequent malignancy in oral cavity, which has become a rapid increasing problem for the global public health with unclear molecular mechanism. Previously, Tiam1 (T-lymphoma invasion and metastasis inducing factor 1) has been reported as a potential oncogene for OSCC. Here, we in-depth explored its signaling mechanism for the disorder. The mRNA and protein expression levels of primary differentially expressed genes (Tiam1, Fibulin-3, and MMP-7) were measured in different TNM stages of OSCC patients using RT-PCR and ELISA methods. Based on the analysis of human OSCC cell line CAL27, the relationships between these factors have been further investigated and the interactions were also examined. The luciferase reporter assay was established for the promoter region of MMP-7. Both the epithelial (E-cadherin) and mesenchymal protein markers (Vimentin and Snail) expressions were examined using western blotting. The mRNA and protein activities of Fibulin-3 declined as the increase of TNM stage. Inversely, the mRNA and protein levels of Tiam1 and MMP-7 elevated significantly as OSCC progressed. Tiam1 transfection in CAL27 cells stimulated the expression of MMP-7 by accelerating the nuclear translocation of β-catenin, which was opposite to the functions of Fibulin-3. Moreover, Tiam1 interacted directly with Fibulin-3. The Tiam1 induced OSCC epithelial-mesenchymal transition (EMT) via MMP-7 activation, which was dependent on the direct binding of β-catenin at the promoter region. Collectively, these results indicated that Tiam1 competed with Fibulin-3 for nuclear β-catenin translocation, which subsequently stimulated MMP-7 expression by TCF-4 domain interaction following EMT initiation in OSCC development. Our systematical work hypothesized an innovative signaling cassette for OSCC progression, which provided beneficial references for future clinical study.





Similar content being viewed by others
References
Rivera C. Essentials of oral cancer. Int J Clin Exp Pathol. 2015;8(9):11884–94.
Panarese I, Aquino G, Ronchi A, et al. Oral and oropharyngeal squamous cell carcinoma: prognostic and predictive parameters in the etiopathogenetic route. Expert Rev Anticancer Ther. 2019;19(2):105–19.
Johnson DE, Burtness B, Leemans CR, et al. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6(1):92.
Afzali P, Ward BB. Management of the neck in oral squamous cell carcinoma: background, classification, and current philosophy. Oral Maxillofac Surg Clin North Am. 2019;31(1):69–84.
Livingstone I, Uversky VN, Furniss D, et al. The pathophysiological significance of Fibulin-3. Biomolecules. 2020;10(9):1294.
Zhang C, Yu C, Li W, et al. Fibulin-3 affects vascular endothelial function and is regulated by angiotensin II. Microvasc Res. 2020;132:104043.
Wang X, Zhang Q, Li C, et al. Fibulin-3 has anti-tumorigenic activities in cutaneous squamous cell carcinoma. J Invest Dermatol. 2019;139(8):1798-1808 e1795.
Ledda C, Caltabiano R, Vella F, et al. Fibulin-3 as biomarker of malignant mesothelioma. Biomark Med. 2019;13(10):875–86.
Chen X, Meng J, Yue W, et al. Fibulin-3 suppresses Wnt/beta-catenin signaling and lung cancer invasion. Carcinogenesis. 2014;35(8):1707–16.
Diamantopoulou Z, White G, Fadlullah MZH, et al. TIAM1 antagonizes TAZ/YAP Both in the destruction complex in the cytoplasm and in the nucleus to inhibit invasion of intestinal epithelial cells. Cancer Cell. 2017;31(5):621-634 e626.
Ding M, Li Y, Yang Y, et al. Elevated expression of Tiam1 is associated with poor prognosis and promotes tumor progression in pancreatic cancer. Onco Targets Ther. 2018;11:4367–75.
Nomden M, Beljaars L, Verkade HJ, et al. Current concepts of biliary atresia and matrix metalloproteinase-7: a review of literature. Front Med (Lausanne). 2020;7:617261.
Chai AWY, Lim KP, Cheong SC. Translational genomics and recent advances in oral squamous cell carcinoma. Semin Cancer Biol. 2020;61:71–83.
Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
Moskovitz J, Moy J, Ferris RL. Immunotherapy for head and neck squamous cell carcinoma. Curr Oncol Rep. 2018;20(2):22.
Wu QY, Wang Y, Tong JC, et al. Expression and clinical significance of Tiam1 gene in esophageal carcinoma. Int J Clin Exp Med. 2015;8(11):21229–34.
Shan G, Tang T, Qian H, et al. Expression of Tiam1 and Rac1 proteins in renal cell carcinoma and its clinical-pathological features. Int J Clin Exp Pathol. 2017;10(11):11114–21.
Liu L, Wu B, Cai H, et al. Tiam1 promotes thyroid carcinoma metastasis by modulating EMT via Wnt/beta-catenin signaling. Exp Cell Res. 2018;362(2):532–40.
Boissier P, Huynh-Do U. The guanine nucleotide exchange factor Tiam1: a Janus-faced molecule in cellular signaling. Cell Signal. 2014;26(3):483–91.
Winn RA, Bremnes RM, Bemis L, et al. gamma-Catenin expression is reduced or absent in a subset of human lung cancers and re-expression inhibits transformed cell growth. Oncogene. 2002;21(49):7497–506.
Lim JH, Park JW, Chun YS. Human arrest defective 1 acetylates and activates beta-catenin, promoting lung cancer cell proliferation. Cancer Res. 2006;66(22):10677–82.
Saitoh M. Involvement of partial EMT in cancer progression. J Biochem. 2018;164(4):257–64.
Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118(3):277–9.
Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–39.
Zhang Q, Liu S, Parajuli KR, et al. Interleukin-17 promotes prostate cancer via MMP7-induced epithelial-to-mesenchymal transition. Oncogene. 2017;36(5):687–99.
Funding
This research was funded by the National Natural Science Foundation of China (Grant NO.82070687 to Bo Zhang).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, H., Ji, Y., Zhang, B. et al. Fibulin-3 sponges Tiam1 to manipulate MMP-7 activity through β-catenin signaling in oral squamous cell carcinoma. Med Oncol 39, 154 (2022). https://doi.org/10.1007/s12032-022-01746-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01746-4