Abstract
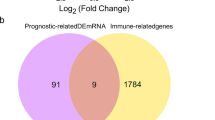
Most colorectal cancer (CRC) patients are diagnosed with advanced stages and low prognosis. We aimed to identify potential diagnostic and prognostic biomarkers, as well as active small molecules of CRC. Microarray data (GSE9348, GSE35279, and GSE106582) were obtained from the Gene Expression Omnibus database. Differentially expressed genes (DEGs) were identified by the GEO2R platform. Common DEGs were selected for Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis. Cytoscape software was used to construct protein–protein interaction networks and identify hub genes. Hub genes were evaluated by Kaplan–Meier survival analysis in the GEPIA database and validated in two independent microarray data (GSE74602 and GSE83889). Common DEGs were used to select active small molecules by the connectivity map database. A total of 166 DEGs were identified as common DEGs. GO analysis demonstrated that common DEGs were significantly enriched in the apoptotic process, cell proliferation, and cell adhesion. KEGG analysis indicated that the most enriched pathways were the PI3K-Akt signaling pathway and extracellular matrix-receptor interaction. COL1A2, THBS2, TIMP1, and CXCL8 significantly upregulated in colorectal tumor. High expressions of COL1A2, THBS2, and TIMP1 were associated with poor survival, while high expressions of CXCL8 were associated with better survival. We selected 11 small molecules for CRC therapy. In conclusion, we found key dysregulated genes associated with CRC and potential small molecules to reverse them. COL1A2, THBS2, TIMP1, and CXCL8 may act as diagnostic and prognostic biomarkers of CRC.





Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available in the Gene Expression Omnibus repository (https://www.ncbi.nlm.nih.gov/geo/), GEPIA repository (https://gepia.cancer-pku.cn/detail.php), and connectivity map database (https://portals.broadinstitute.org/CMap/).
Abbreviations
- CRC:
-
Colorectal cancer
- GEO:
-
Gene Expression Omnibus
- DEG:
-
Differentially expressed genes
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- PPI:
-
Protein–protein interaction
- COL1A2:
-
Collagen type I alpha 2 chain
- THBS2:
-
Thrombospondin 2
- CXCL8:
-
C-X-C motif chemokine ligand 8
- TIMP1:
-
TIMP metallopeptidase inhibitor 1
- CMap:
-
Connectivity map
- logFC:
-
Log fold change
- DAVID:
-
Database for Annotation Visualization and Integrated Discovery
- BP:
-
Biological processes
- CC:
-
Cellular components
- MF:
-
Molecular functions
- MCC:
-
Maximum Correlation Criteria
- ECM:
-
Extracellular matrix
References
Ferlay J, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–53.
Rossi, M, et al. Colorectal cancer and alcohol consumption—populations to molecules. Cancers (Basel). 2018;10(2):38.
Hofseth LJ, et al. Early-onset colorectal cancer: initial clues and current views. Nat Rev Gastroenterol Hepatol. 2020;17(6):352–64.
Koncina E, et al. Prognostic and Predictive Molecular Biomarkers for Colorectal Cancer: Updates and Challenges. Cancers (Basel). 2020;12(2):319.
Carethers JM, Jung BH. Genetics and Genetic Biomarkers in Sporadic Colorectal Cancer. Gastroenterology. 2015;149(5):1177–90.
Liu YR, et al. Neurexophilin and PC-esterase domain family member 4 (NXPE4) and prostate androgen-regulated mucin-like protein 1 (PARM1) as prognostic biomarkers for colorectal cancer. J Cell Biochem. 2019;120(10):18041–52.
Xie XJ, et al. The Generation and Validation of a 20-Genes Model Influencing the Prognosis of Colorectal Cancer. J Cell Biochem. 2017;118(11):3675–85.
Sun G, et al. Identification of a five-gene signature with prognostic value in colorectal cancer. J Cell Physiol. 2019;234(4):3829–36.
Wan L, et al. HOTAIRM1 as a potential biomarker for diagnosis of colorectal cancer functions the role in the tumour suppressor. J Cell Mol Med. 2016;20(11):2036–44.
Wang W, et al. Gene selection for the discrimination of colorectal cancer. Curr Mol Med. 2020;20(6):415–28.
Giopanou I, Pintzas A. RAS and BRAF in the foreground for non-small cell lung cancer and colorectal cancer: Similarities and main differences for prognosis and therapies. Crit Rev Oncol Hematol. 2020;146:102859.
Smoot ME, et al. Cytoscape 28 new features for data integration and network visualization. Bioinformatics. 2011;27(3):431–2.
Chin C, et al. Cytohubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8(Suppl 4):S11.
Tang Z, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:98–102.
Lamb J. The Connectivity Map: a new tool for biomedical research. Nat Rev Cancer. 2007;7(1):54–60.
Wu D, Wang X. Application of clinical bioinformatics in lung cancer-specific biomarkers. Cancer Metastasis Rev. 2015;34(2):209–16.
Liang Y, et al. Gene expression profiling reveals molecularly and clinically distinct subtypes of glioblastoma multiforme. Proc Natl Acad Sci U S A. 2005;102(16):5814–9.
Bahrami A, et al. Therapeutic Potential of Targeting PI3K/AKT Pathway in Treatment of Colorectal Cancer: Rational and Progress. J Cell Biochem. 2018;119(3):2460–9.
Ishaque N, et al. Whole genome sequencing puts forward hypotheses on metastasis evolution and therapy in colorectal cancer. Nat Commun. 2018;9(1):4782.
Stelzl U, et al. A human protein-protein interaction network: a resource for annotating the proteome. Cell. 2005;122(6):957–68.
Zou X, et al. Up-regulation of type I collagen during tumorigenesis of colorectal cancer revealed by quantitative proteomic analysis. J Proteomics. 2013;94:473–85.
Rodia MT, et al. LGALS4, CEACAM6, TSPAN8, and COL1A2: Blood Markers for Colorectal Cancer-Validation in a Cohort of Subjects With Positive Fecal Immunochemical Test Result. Clin Colorectal Cancer. 2018;17(2):217–28.
Yu Y, et al. The inhibitory effects of COL1A2 on colorectal cancer cell proliferation, migration, and invasion. J Cancer. 2018;9(16):2953–62.
Ao R, et al. Silencing of COL1A2, COL6A3, and THBS2 inhibits gastric cancer cell proliferation, migration, and invasion while promoting apoptosis through the PI3k-Akt signaling pathway. J Cell Biochem. 2018;119(6):4420–34.
Slattery ML, et al. The PI3K/AKT signaling pathway: Associations of miRNAs with dysregulated gene expression in colorectal cancer. Mol Carcinog. 2018;57(2):243–61.
Tian Q, et al. THBS2 is a biomarker for AJCC stages and a strong prognostic indicator in colorectal cancer. J BUON. 2018;23(5):1331–6.
Sun XY, et al. MiR-93-5p promotes cervical cancer progression by targeting THBS2/MMPS signal pathway. Eur Rev Med Pharmacol Sci. 2019;23(12):5113–211.
Lambert E, et al. TIMPs as multifacial proteins. Crit Rev Oncol Hematol. 2004;49(3):187–98.
Song G, et al. TIMP1 is a prognostic marker for the progression and metastasis of colon cancer through FAK-PI3K/AKT and MAPK pathway. J Exp Clin Cancer Res. 2016;35(1):148.
Pellegrini P, et al. Simultaneous measurement of soluble carcinoembryonic antigen and the tissue inhibitor of metalloproteinase TIMP1 serum levels for use as markers of pre-invasive to invasive colorectal cancer. Cancer Immunol Immunother. 2000;49(7):388–94.
Eckfeld C, et al. Functional disparities within the TIMP family in cancer: hints from molecular divergence. Cancer Metastasis Rev. 2019;38(3):469–81.
Prokopchuk O, et al. Elevated systemic levels of the matrix metalloproteinase inhibitor TIMP-1 correlate with clinical markers of cachexia in patients with chronic pancreatitis and pancreatic cancer. BMC Cancer. 2018;18(1):128.
Dabkeviciene D, et al. The role of interleukin-8 (CXCL8) and CXCR2 in acquired chemoresistance of human colorectal carcinoma cells HCT116. Med Oncol. 2015;32(12):258.
Pączek S, et al. CXCL-8 in Preoperative Colorectal Cancer Patients: Significance for Diagnosis and Cancer Progression. Int J Mol Sci. 2020;21(6):2040.
Ha H, Debnath B, Neamati N. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics. 2017;7(6):1543–88.
Lee YS, et al. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br J Cancer. 2012;106(11):1833–41.
Ning Y, et al. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer. 2011;128(9):2038–49.
Moody TW, et al. VIP-ellipticine derivatives inhibit the growth of breast cancer cells. Life Sci. 2002;71(9):1005–144.
Duan C, et al. Piperlongumine induces gastric cancer cell apoptosis and G2/M cell cycle arrest both in vitro and in vivo. Tumour Biol. 2016;37(8):10793–804.
Hall JA, et al. Novobiocin Analogues That Inhibit the MAPK Pathway. J Med Chem. 2016;59(3):925–33.
Al-Saeedi FJ. Study of the cytotoxicity of asiaticoside on rats and tumour cells. BMC Cancer. 2014;14:220.
Eckschlager T, et al. Histone Deacetylase Inhibitors as Anticancer Drugs. Int J Mol Sci. 2017;18(7):1414.
Lu C, Wang W, El-Deiry WS. Non-genotoxic anti-neoplastic effects of ellipticine derivative NSC176327 in p53-deficient human colon carcinoma cells involve stimulation of p73. Cancer Biol Ther. 2008;7(12):2039–46.
Kuo YC, et al. Ellipticine induces apoptosis through p53-dependent pathway in human hepatocellular carcinoma HepG2 cells. Life Sci. 2006;78(22):2550–7.
Martinkova E, et al. alpha5beta1 integrin antagonists reduce chemotherapy-induced premature senescence and facilitate apoptosis in human glioblastoma cells. Int J Cancer. 2010;127(5):1240–8.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31371290), the Frontier and Key Technology Innovation Project of Guangdong Province (No.2014B010118003), and the Science and Technology Planning Project of Guangdong Province (No. 2015B010129008).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. DEG identification and gene functional enrichment analysis were performed by JX and LX. The PPI network construction and hub genes verification were performed by MG, LQ, CD, and ZL. The active small molecules selection was finished by YW, JX, and QW. The first draft of the manuscript was written by ZZ, XL, and WW. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12032_2020_1425_MOESM1_ESM.tif
Supplementary file1 Supplementary Fig. 1 Venn diagram of the common DEGs among GSE9348, GSE35279 and GSE106582. a 78 common upregulated genes. b 88 common downregulated genes (TIF 11085 kb)
Rights and permissions
About this article
Cite this article
Zheng, Z., Xie, J., Xiong, L. et al. Identification of candidate biomarkers and therapeutic drugs of colorectal cancer by integrated bioinformatics analysis. Med Oncol 37, 104 (2020). https://doi.org/10.1007/s12032-020-01425-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-020-01425-2