Abstract
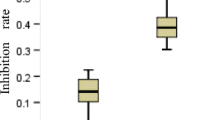
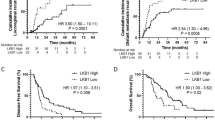
Prior studies have demonstrated an association between excision repair cross-complementation group 1 (ERCC1) expression level and outcomes in patients with advanced non-small cell lung cancer (NSCLC) treated with platinum-based chemotherapy. The aim of this study was to assess the impact of ERCC1 on survival for patients with stage IIIB/IV non-squamous NSCLC (NS-NSCLC) enrolled in the INNOVATIONS trial, thus receiving as treatment either erlotinib/bevacizumab (EB) or cisplatin/gemcitabine/bevacizumab (PGB). We retrospectively analyzed tumor tissue of 72 patients using immunohistochemistry to assess the expression of ERCC1. The distribution between treatment arms was equal (36 patients each). Two different H scores were calculated and correlated with survival. In ERCC1-positive patients, no significant difference in terms of progression-free survival (PFS) between treatment arms has been detected. ERCC1-negative patients benefited from PGB compared to EB arm (H score: HR = 0.377, 95% CI [0.167–0.849], p = 0.0151; modified H score: HR = 0.484, 95% CI [0.234–1.004], p = 0.0468). With respect to the scoring system, in the EB-arm, a significant superior PFS turned out in ERCC1-positive patients when employing the H-score (HR = 0.430, 95% CI [0.188–0.981], p = 0.0397; median 4.9 vs. 3.9 months), but not with the modified H-score. Our findings support the hypothesis that NS-NSCLC displaying a low ERCC1 expression might benefit from cisplatin-based chemotherapy. High expression indicated better PFS in the EB arm supporting the prognostic impact. However, as impact of ERCC1-assessment even might depend on scoring systems differences, the need in standardization of assessment methodology is emphasized.


Similar content being viewed by others
References
Duruisseaux M, Besse B, Cadranel J, Pérol M, Mennecier B, Bigay-Game L, Descourt R, Dansin E, Audigier-Valette C, Moreau L, Hureaux J, Veillon R, Otto J, Madroszyk-Flandin A, Cortot A, Guichard F, Boudou-Rouquette P, Langlais A, Missy P, Morin F, Moro-Sibilot D. Overall survival with crizotinib and next generation ALK-inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINAKL): a French nationwide cohort retrospective study. Oncotarget 2017;8(13):21903–17. https://doi.org/10.18632/oncotarget.15746.
Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland Å, Giannone V, D’Amelio AM Jr, Zhang P, Mookerjee B, Johnson BE. Dabrafenib plus trametinib in patients with previously untreated BRAF V600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017;18(10):1307–16. https://doi.org/10.1016/S1470-2045(17)30679-4.
Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Tsuji F, Linke R, Rosell R, Corral J, Migliorino MR, Pluzanski A, Sbar EI, Wang T, White JL, Nadanaciva S, Sandin R, Mok TS. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(11):1454–66. https://doi.org/10.1016/S1470-2045(17)30608-3.
Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, Riely GJ, Varella-Garcia M, Shapiro GI, Costa DB, Doebele RC, Le LP, Zheng Z, Tan W, Stephenson P, Shreeve SM, Tye LM, Christensen JG, Wilner KD, Clark JW, Iafrate AJ. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371(21):1963–71. https://doi.org/10.1056/NEJMoa1406766.
Shea M, Costa DB, Rangachari D. Management of advanced non-small cell lung cancers with known mutations or rearrangements: latest evidence and treatment approaches. Ther Adv Respir. 2016;10(2):113–29.
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33.
Reed E. ERCC1 and clinical resistance to platinum-based therapy. Clin Cancer Res. 2005;11:6100–2.
Simon GR, Sharma S, Cantor A, Smith P, Bepler G. ERCC1 expression is a predictor of survival in resected patients with non-small cell lung cancer. Chest 2005;127:978–83.
Olaussen KA, Dunant A, Fouret P, Brambilla E, Andre F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH, Stahel R, Sabatier L, Pignon J-P, Tursz T, Le Chevalier T, Soria J-C. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–91.
Friboulet L, Olaussen KA, Pignon J-P, Shepherd FA, Tsao M-S, Graziano S, Kratzke R, Douillard J-Y, Seymour L, Pirker R, Filipits M, Andre F, Solary E, Ponsonnailles F, Robin A, Stoclin A, Dorvault N, Commo F, Adam J, Vanhecke E, Saulnier P, Thomale J, Le Chevalier T, Dunant A, Rousseau V, Le Teuff G, Brambilla E, Soria J-C. ERCC1 isoform expression and DNA repair in non-small-cell lung cancer. N Engl J Med. 2013;368:1101–10.
Thomas M, Fischer J, Andreas S, Kortsik C, Grah C, Serke M, von Eiff M, Witt C, Kollmeier J, Müller E, Schenk M, Schröder M, Villalobos M, Reinmuth N, Penzel R, Schnabel P, Acker T, Reuss A, Wolf M. Erlotinib and bevacizumab versus cisplatin, gemcitabine nd bevacizumab in unselected nonsquamous nonsmall cell lung cancer. Eur Respir J. 2015;46(1):219–29.
Holm B, Mellemgaard A, Skov T, Guldhammer Skov B. Different impact of excision repair cross-complementation group 1 on survival in male and female patients with inoperable non-small-cell lung cancer treated with carboplatin and gemcitabine. J Clin Oncol. 2009;27:4254–9.
Lord RVN, Brabender J, Gandara D, Alberola V, Camps C, Domine M, Cardenal F, Sanchez JM, Gumerlock PH, Taron M, Sanchez JJ, Danenberg KD, Danenberg PV, Rosell R. Low ERCC1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clin Cancer Res. 2002;8:2286–91.
Roth JA, Carlson JJ. Prognostic role of ERCC1 in advanced non-small-cell lung cancer: a systematic review and meta-analysis. Clin Lung Cancer 2011;12(6):393–401.
Selvakumaran M, Pisarcik DA, Bao R, et al. Enhanced cisplatin toxicity by disturbing the nucleotide excision excision repair pathway in ovarian cancer cell lines. Cancer Res. 2003;63:1311–6.
Chang IY, Kim MH, Kim HB, et al. Small interfering RNA-induced suppression of ERCC1 enhances sensitivity of human cancer cells to cisplatin. Biochem Biophys Res Commun. 2005;327:225–33.
Ren S, Zhou S, Zhang L, et al. High-level mRNA of excision repair cross-complementation group 1 gene is associated with poor outcome of platinum-based doublet chemotherapy of advanced nonsmall cell lung cancer patients. Cancer Investig. 2010;28:1078–83.
Lee HW, Choi YW, Han JH, et al. Expression of excision repair cross-complementation group 1 protein predicts poor outcome in advanced non-small cell lung cancer patients treated with platinum-based doublet chemotherapy. Lung Cancer 2009;65:377–82.
Hubner RA, Riley RD, Billingham LJ, Popat S. Excision repair cross-complementation group 1 (ERCC1) status and lung cancer outcomes: a meta-analysis of published studies and recommendations. PLoS ONE 2011;6(10):e25164.
Bepler G, Williams C, Schell MJ, Chen W, Zheng Z, Simon G, Gadgeel S, Zhao X, Schreiber F, Brahmer J, Chiappori A, Tanvetyanon T, Pinder-Scheck M, Gray J, Haura E, Antonia S, Fischer JR. Randomized international phase III trial of ERCC1 and RRM1 expression-based chemotherapy versus gemcitabine/carboplatin in advanced non-small-cell lung cancer. J Clin Oncol. 2013;31:2404–12.
Lee SM, Falzon M, Blackhall F, Spicer J, Nicolson M, Chaudhuri A, Middleton G, Ahmed S, Hicks J, Crosse B, Napier M, Singer JM, Ferry D, Lewanski C, Forster M, Rolls SA, Capitanio A, Rudd R, Iles N, Ngai Y, Gandy M, Lillywhite R, Hackshaw A. Randomized prospective biomarker trial of ERCC1 for comparing platinum and nonplatinum therapy in advanced non-small-cell lung cancer: ERCC1 trial (ET). J Clin Oncol. 2017;35:402–11.
Taillade L, Penault-Llorca F, Boulet T, Fouret P, Michiels S, Taranchon E, Mountzios G, Validire P, Domont J, Girard P, Grunenwald D, Le Chevalier T, Soria JC. Immunohistochemical expression of biomarkers: a comparative study between diagnostic bronchial biopsies and surgical specimens of non-small-cell lung cancer. Ann Oncol. 2007;18:1042–50.
Choi CM, Yang SC, Jo HJ, Song SY, Jeon YJ, Jang TW, et al. Proteins involved in DNA damage response pathways and survival of stage I non-small cell lung cancer. Ann Oncol. 2012;23:2088–93.
Piercall WE, Olaussen KA, Rousseau V, Brambilla E, Sprott KM, Andre F, et al. Cisplatin benefit is predicted by immunohistochemical analysis of DNA repair proteins in squamous cell carcinoma but not adenocarcinoma: theranostic modeling by NSCLC constituent histological subclasses. Ann Oncol. 2012;23:2245–52.
Muley TR, Sianidou M, Thomas M, Bischoff H, Dienemann H, Meister M, Schneider MA, Schnabel PA, Warth A. Comparison of two ERCC1 antibodies as prognostic and predictive biomarkers for early non-small cell lung cancer. Anticancer Res. 2014;34:3707–14.
Postel-Vinay S, Bajrami I, Friboulet L, Elliott R, Fontebasso Y, Dorvault N, Olaussen KA, Andre F, Soria J-C, Lord CJ, Ashworth A. A high-throughput screen identifies PARP1/2 inhibitors as a potential therapy for ERCC1-deficient non-small cell lung cancer. Oncogene 2013;32:5377–87.
Acknowledgements
INNOVATIONS has been supported by Roche Germany, which provided erlotinib, bevacizumab, and a Grant for conducting the study and the associated biomarker analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MT has received Honoraria (Grant) from Roche. All other authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Villalobos, M., Czapiewski, P., Reinmuth, N. et al. ERCC1 assessment in upfront treatment with and without cisplatin-based chemotherapy in stage IIIB/IV non-squamous non-small cell lung cancer. Med Oncol 35, 106 (2018). https://doi.org/10.1007/s12032-018-1169-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-018-1169-5