Abstract
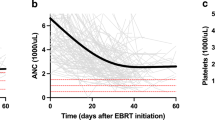
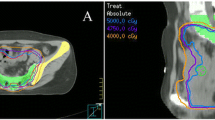
The aim of the study was to model acute hematologic toxicity (HT) and dose to pelvic osseous structures in anal cancer patients treated with definitive chemo-radiation (CT-RT). A total of 53 patients receiving CT-RT were analyzed. Pelvic bone marrow and corresponding subsites were contoured: ilium, lower pelvis and lumbosacral spine (LSBM). Dose-volume histograms points and mean doses were collected. Logistic regression was performed to correlate dosimetric parameters and ≥G3 HT as endpoint. Normal tissue complication probability (NTCP) was evaluated with the Lyman-Kutcher-Burman (LKB) model. Logistic regression showed a significant correlation between LSBM-mean dose and ≥G3 leukopenia (β coefficient 0.122; p = 0.030; 95% CI 0.012–0.233). According to NTCP modeling, the predicted HT probability had the following parameters: TD50: 37.5 Gy, γ 50: 1.15, m: 0.347. For node positive patients, TD50: 35.2 Gy, γ 50: 2.27, m: 0.176 were found. Node positive patients had significantly higher PBM-V15 (Mean 81.1 vs. 86.7%; p = 0.04), -V20 (Mean 72.7 vs. 79.9%; p = 0.01) and V30 (Mean 50.2 vs. 57.3%; p = 0.03). Patients with a mean LSBM dose >32 Gy had a 1.81 (95% CI 0.81–4.0) relative risk to develop ≥G3 leukopenia. For node positive patients, those risks were 2.67 (95% CI 0.71–10). LKB modeling seems to suggest that LSBM-mean dose should be kept below 32 Gy to minimize ≥G3 HT in anal cancer patients treated with IMRT and concurrent chemotherapy. The contribution of LSBM dose in the development of HT above 25 Gy seems steeper in node positive patients.


Similar content being viewed by others
References
UKCCCR Anal cancer Trial Working Party. UK Co-ordination Committee on Cancer Research: epidermoid anal cancer: results from the UKCCCR randomized trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. Lancet. 1996;348:1049–54.
Bartelink H, Roelofsen F, Eschwege F, Rougier P, Bosset JF, Gonzalez DG, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Group. J Clin Oncol. 1997;15:2040–9.
Gunderson LL, Winter KA, Ajani JA, Pedersen JE, Moughan J, Benson AB 3rd, et al. Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol. 2012;30:4344–51.
Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB 3rd, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008;299:1914–21.
Franco P, Mistrangelo M, Arcadipane F, Munoz F, Sciacero P, Spadi R, et al. Intensity-modulated radiation therapy with simultaneous integrated boost combined with concurrent chemotherapy for the treatment of anal cancer patients: 4-year results of a consecutive case series. Cancer Invest. 2015;33:259–66.
Mauch P, Constine L, Greenberger J, Knospe W, Sullivan J, Liesveld JL, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1319–39.
Kachnic LA, Winter K, Myerson RJ, Goodyear MD, Willins J, Esthappan J, et al. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mytomycin C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2013;86:27–33.
Emami B, Lyman J, Borwn A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–22.
Bazan JG, Luxton G, Kozak MM, Anderson EM, Hancock SL, Kapp DS, et al. Impact of chemotherapy on normal tissue complication probability models of acute hematologic toxicity in patients receiving pelvic intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2012;87:983–91.
Mell LK, Kochanski JD, Roeske JC, Haslam JJ, Mehta N, Yamada SD, et al. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:1356–65.
Wan J, Liu K, Li K, Li G, Zhang Z. Can dosimetric parameters predict acute hematologic toxicity in rectal cancer patients treated with intensity-modulated pelvic radiotherapy? Radiat Oncol. 2015;10:162.
Kachnic LA, Tsai HK, Coen JJ, Blaszkowsky LS, Hartshorn K, Kwak EL, et al. Dose-painted intensity-modulated radiation therapy for anal cancer: a multi-institutional report of acute toxicity and response to therapy. Int J Radiat Oncol Biol Phys. 2012;82:153–8.
Radiation Therapy Oncology group. Acute radiation morbidity scoring criteria. http://www.rtog.org. Accessed 16th Sept 2015.
Mell LK, Schomas DA, Salama JK, Devisetty K, Aydogan B, Miller RC, et al. Association between bone marrow dosimetric parameters and acute hematologic toxicity in anal cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:1431–7.
Bentzen SM, Tucker SL. Quantifying the position and steepness of radiation dose-response curves. Int J Radiat Biol. 1997;71:531–42.
Dasu A, Toma-Dasu I, Fowler JF. Should single or distributed parameters be used to explain the steepness of tumor control probability curves? Phys Med Biol. 2003;48:387–97.
Bazan JG, Luxton G, Mok EC, Koong AC, Chang DT. Normal tissue complication probability modeling of acute hematological toxicity in patients treated with intensity-modulated radiation therapy for squamous cell carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2012;84:700–6.
Flam M, John M, Pajak TF, et al. Role of mytomicin in combination with fluorouracil and radiotherapy, and salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527–39.
Salama J, Mell LK, Schomas DA, Miller RC, Devisetty K, Jani AB, et al. Concurrent chemotherapy and intensity modulated radiation therapy for anal cancer patients: a multicenter experience. J Clin Oncol. 2007;25:4581–6.
Ellis RE. The distribution of active bone marrow in the adult. Phys Med Biol. 1961;5:255–8.
Liang Y, Messer K, Rose BS, Rose BS, Lewis JH, Jiang SB, et al. Impact of bone marrow radiation dose on acute hematologic toxicity in cervical cancer: principal component analysis on high dimensional data. Int J Radiat Oncol Biol Phys. 2010;78:912–9.
Cheng JC, Bazan JG, Wu JK, Koong AC, Chang DT. Lumbosacral spine and marrow cavity modeling of acute hematologic toxicity in patients treated with intensity modulated radiation therapy for squamous cell carcinoma of the anal canal. Pract Radiat Oncol. 2014;4:198–206.
Albuquerque K, Giangreco D, Morrison C, Siddiqui M, Sinacore J, Potkul R, et al. Radiation-related predictors of hematologic toxicity after concurrent chemoradiation for cervical cancer and implications for bone marrow-sparing pelvic IMRT. Int J Radiat Oncol Biol Phys. 2011;79:1043–7.
Rose BS, Aydogan B, Liang Y, Liang Y, Yeginer M, Hasselle MD, et al. Normal tissue complication probability modeling of acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:800–7.
Zhu H, Zakeri K, Vaida F, Carmona R, Dadachanji KK, Bair R, et al. Longitudinal study of acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. J Med Imaging Radiat Oncol. 2015;59:386–93.
Roeske JC, Lujan A, Reba RC, Penney BC, Yamada DS, Mundt AJ. Incorporation of SPECT bone marrow imaging into intensity modulated whole-pelvic radiation therapy treatment planning for gynecologic malignancies. Radiother Oncol. 2005;77:11–7.
Rose BS, Liang Y, Lau SK, et al. Correlation between radiation dose to 18FDG-PET defined active bone marrow subregions and acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:1185–91.
Franco P, Arcadipane F, Ragona R, Mistrangelo M, Cassoni P, Rondi N, et al. Early-stage node negative (T1-T2N0) anal cancer treated with simultaneous integrated boost radiotherapy and concurrent chemotherapy. Anticancer Res. 2016;36:1943–8.
Franco P, Arcadipane F, Ragona R, Mistrangelo M, Cassoni P, Rondi N, et al. Locally advanced (T3-T4 or N+) anal cancer treated with simultaneous integrated boost radiotherapy and concurrent chemotherapy. Anticancer Res. 2016;36:2027–32.
Franco P, Arcadipane F, Ragona R, Mistrangelo M, Cassoni P, Munoz F, et al. Volumetric modulated arc therapy (VMAT) in the combined modality treatment of anal cancer patients. Br J Radiol. 1060;2016(89):2015832.
Franco P, Ragona R, Arcadipane F, Mistrangelo M, Cassoni P, Rondi N, et al. Dosimetric predictors of acute hematologic toxicity during concurrent intensity-modulated radiotherapy and chemotherapy for anal cancer. Clin Transl Oncol. 2016 (in press). doi:10.1007/s12094-016-1504-2.
Franco P, Arcadipane F, Ragona R, Lesca A, Gallio E, Mistrangelo M, et al. Dose to specific subregions of pelvic bone marrow defined with FDG-PET as a predictor of hematologic nadirs during concomitant chemoradiation in anal cancer patients. Med Oncol. 2016;33:72.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we do not have any conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Franco, P., Ragona, R., Arcadipane, F. et al. Lumbar-sacral bone marrow dose modeling for acute hematological toxicity in anal cancer patients treated with concurrent chemo-radiation. Med Oncol 33, 137 (2016). https://doi.org/10.1007/s12032-016-0852-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-016-0852-7