Abstract
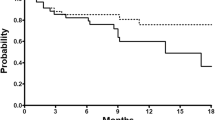
The aim of this paper was to evaluate the activity and tolerability of weekly docetaxel (D) combined with weekly epirubicin (EPI) in patients with advanced castrate-resistant prostate cancer (CRPC) previously exposed to D and abiraterone acetate (AA). Locally advanced or metastatic CRPC patients with 0–2 performance status, who had progressed after D and AA therapy, were included in the study. Previous treatment with chemotherapy agent cabazitaxel was also admitted. Treatment consisted of D 30 mg/m2 intravenously (i.v.) and EPI 30 mg/m2 i.v., every week (D/EPI). Chemotherapy was administered until disease progression or unacceptable toxicity. In our institution, twenty-six patients received D/EPI: their median age was 72 years (range 59–83 years). Twenty-three (88.5 %) patients had bone metastases. A decrease in PSA levels ≥50 % was observed in seven patients (26.9 %, 95 % CI: 0.11–0.47); of these, five had achieved a ≥50 % PSA response during prior first-line D and six had achieved a PSA response during prior AA Among the subjects who were symptomatic at baseline, pain was reduced in nine patients (38.1 %) with a significant decrease in analgesic use. Median progression-free survival was 4.4 months (95 % CI, 3–5.2), and median overall survival was 10.7 months (95 % CI, 8.9–18.4). Treatment was well tolerated and no grade 4 toxicities were observed. Our findings suggest that weekly D/EPI is feasible and active in heavily pretreated advanced CRPC patients and seem to support the hypothesis that the addition of EPI to D may lead to overcome the resistance to D in a subgroup of patients.
Similar content being viewed by others
References
Bedoya DJ, Mitsiades N. Abiraterone acetate, a first-in-class CYP17 inhibitor, establishes a new treatment paradigm in castration-resistant prostate cancer. Expert Rev Anticancer Ther. 2012;12(1):1–3.
Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, Ettinger SL, Gleave ME, Nelson CC. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68(15):6407–15.
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Fléchon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI, COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005.
Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng S, Carles J, Mulders PF, Basch E, Small EJ, Saad F, Schrijvers D, Van Poppel H, Mukherjee SD, Suttmann H, Gerritsen WR, Flaig TW, George DJ, Yu EY, Efstathiou E, Pantuck A, Winquist E, Higano CS, Taplin ME, Park Y, Kheoh T, Griffin T, Scher HI, Rathkopf DE, COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–48.
Harland S, Staffurth J, Molina A, Hao Y, Gagnon DD, Sternberg CN, Cella D, Fizazi K, Logothetis CJ, Kheoh T, Haqq CM, de Bono JS, Scher HI, COU-AA-301 Investigators. Effect of abiraterone acetate treatment on the quality of life of patients with metastatic castration-resistant prostate cancer after failure of docetaxel chemotherapy. Eur J Cancer. 2013;49(17):3648–57.
van Soest RJ, van Royen ME, de Morrée ES, Moll JM, Teubel W, Wiemer EA, Mathijssen RH, de Wit R, van Weerden WM. Cross-resistance between taxanes and new hormonal agents abiraterone and enzalutamide may affect drug sequence choices in metastatic castration-resistant prostate cancer. Eur J Cancer. 2013;49(18):3821–30.
Francini E, Petrioli R, Roviello G. No clear evidence of a clinical benefit of a sequential therapy regimen with abiraterone acetate and enzalutamide. Expert Rev Anticancer Ther. 2014;10:1135–40.
Pezaro CJ, Omlin AG, Altavilla A, Lorente D, Ferraldeschi R, Bianchini D, Dearnaley D, Parker C, de Bono JS, Attard G. Activity of Cabazitaxel in Castration-resistant Prostate Cancer Progressing After Docetaxel and Next-generation Endocrine Agents. Eur Urol. 2013;66(3):459–65.
Al Nakouzi N, Le Moulec S, Albigès L, Wang C, Beuzeboc P, Gross-Goupil M, de La Motte Rouge T, Guillot A, Gajda D, Massard C, Gleave M, Fizazi K, Loriot Y. Cabazitaxel remains active in patients progressing after docetaxel followed by novel androgen receptor pathway targeted therapies. Eur Urol. 2014. doi:10.1016/j.eururo.2014.04.015.
Sella A, Sella T, Peer A, Berger R, Frank SJ, Gez E, Sharide D, Hayat H, Hanovich E, Kovel S, Rosenbaum E, Neiman V, Keizman D. Activity of Cabazitaxel after docetaxel and abiraterone acetate therapy in patients with castration-resistant prostate cancer. Clin Genitourin Cancer. 2014;12(6):428–32.
Oudard S, Kramer G, Caffo O, Creppy L, Loriot Y, Hansen S, Holmberg M, Rolland F, Machiels JP, Krainer M. Docetaxel rechallenge in patients with metastatic castration-resistant prostate cancer. BJU Int. 2014;. doi:10.1111/bju.12845.
Mezynski J, Pezaro C, Bianchini D, Zivi A, Sandhu S, Thompson E, Hunt J, Sheridan E, Baikady B, Sarvadikar A, Maier G, Reid AH, Mulick Cassidy A, Olmos D, Attard G, de Bono J. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: clinical evidence for cross-resistance? Ann Oncol. 2012;23(11):2943–7.
Schweizer MT, Zhou XC, Wang H, Bassi S, Carducci MA, Eisenberger MA, Antonarakis ES. The influence of prior abiraterone treatment on the clinical activity of docetaxel in men with metastatic castration-resistant prostate cancer. Eur Urol. 2014;66(4):646–52.
Suzman DL, Luber B, Schweizer MT, Nadal R, Antonarakis ES. Clinical activity of enzalutamide versus docetaxel in men with castration-resistant prostate cancer progressing after abiraterone. Prostate. 2014;74(13):1278–85.
Petrioli R, Paolelli L, Francini E, Manganelli A, Salvestrini F, Francini G. Weekly docetaxel and epirubicin in treatment of advanced hormone-refractory prostrate cancer. Urology. 2007;69:142–6.
Petrioli R, Pascucci A, Conca R, Chiriacò G, Francini E, Bargagli G, Fiaschi AI, Manganelli A, De Rubertis G, Barbanti G, Ponchietti R, Francini G. Docetaxel and epirubicin compared with docetaxel and prednisone in advanced castrate-resistant prostate cancer: a randomised phase II study. Br J Cancer. 2011;104(4):613–9.
Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, Figg WD, Freidlin B, Halabi S, Hudes G, Hussain M, Kaplan R, Myers C, Oh W, Petrylak DP, Reed E, Roth B, Sartor O, Scher H, Simons J, Sinibaldi V, Small EJ, Smith MR, Trump DL, Wilding G. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–7.
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small EJ, Beer TM, Wilding G, Martin A, Hussain M, Prostate Cancer Clinical Trials Working Group. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–99.
Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0. Published August 9, 2006. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_30. Accessed 14 Sept 2010.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guideline). Prostate Cancer. Version 2.2014. Available at: http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed 13 July 2013.
Horwich A, Hugosson J, de Reijke T, Wiegel T, Fizazi K, Kataja V, Panel Members; European Society for Medical Oncology. Prostate cancer: ESMO Consensus Conference Guidelines 2012. Ann Oncol. 2013;24:1141–62.
Buonerba C, Federico P, Bosso D, Puglia L, Policastro T, Izzo M, Perri F, Vittoria Scarpati GD, Ferro M, Cobelli OD, De Placido S, Aieta M, Imbimbo C, Longo N, Di Lorenzo G. Carboplatin plus etoposide in heavily pretreated castration-resistant prostate cancer patients. Future Oncol. 2014;10(8):1353–60.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12.
Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20.
Caffo O, Pappagallo G, Brugnara S, Caldara A, di Pasquale MC, Ferro A, Frisinghelli M, Murgia V, Russo LM, Soini B, Valduga F, Veccia A, Galligioni E. Multiple rechallenges for castration-resistant prostate cancer patients responding to first-line docetaxel: assessment of clinical outcomes and predictive factors. Urology. 2012;79(3):644–9.
de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, Roessner M, Gupta S, Sartor AO, TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54.
Heidenreich A, Bracarda S, Mason M, Ozen H, Sengelov L, Van Oort I, Papandreou C, Fossa S, Hitier S, Climent MA, European investigators. Safety of cabazitaxel in senior adults with metastatic castration-resistant prostate cancer: results of the European compassionate-use programme. Eur J Cancer. 2014;50(6):1090–9. doi:10.1016/j.ejca.2014.01.006.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petrioli, R., Roviello, G., Fiaschi, A.I. et al. Rechallenge of docetaxel combined with epirubicin given on a weekly schedule in advanced castration-resistant prostate cancer patients previously exposed to docetaxel and abiraterone acetate: a single-institution experience. Med Oncol 32, 52 (2015). https://doi.org/10.1007/s12032-015-0485-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-015-0485-2