Abstract
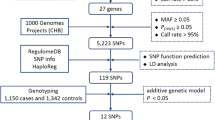
Previous studies have demonstrated that circadian negative feedback loop genes play an important role in the development and progression of many cancers. However, the associations between single-nucleotide polymorphisms (SNPs) in these genes and the clinical outcomes of hepatocellular carcinoma (HCC) after surgical resection have not been studied so far. Thirteen functional SNPs in circadian genes were genotyped using the Sequenom iPLEX genotyping system in a cohort of 489 Chinese HCC patients who received radical resection. Multivariate Cox proportional hazards model and Kaplan–Meier curve were used for the prognosis analysis. Cumulative effect analysis and survival tree analysis were used for the multiple SNPs analysis. Four individual SNPs, including rs3027178 in PER1, rs228669 and rs2640908 in PER3 and rs3809236 in CRY1, were significantly associated with overall survival (OS) of HCC patients, and three SNPs, including rs3027178 in PER1, rs228729 in PER3 and rs3809236 in CRY1, were significantly associated with recurrence-free survival (RFS). Moreover, we observed a cumulative effect of significant SNPs on OS and RFS (P for trend < 0.001 for both). Survival tree analysis indicated that wild genotype of rs228729 in PER3 was the primary risk factor contributing to HCC patients’ RFS. Our study suggests that the polymorphisms in circadian negative feedback loop genes may serve as independent prognostic biomarkers in predicting clinical outcomes for HCC patients who received radical resection. Further studies with different ethnicities are needed to validate our findings and generalize its clinical utility.



Similar content being viewed by others
References
Kondratov RV, Gorbacheva VY, Antoch MP. The role of mammalian circadian proteins in normal physiology and genotoxic stress responses. Curr Top Dev Biol. 2007;78:173–216. doi:10.1016/S0070-2153(06)78005-X.
Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, et al. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288(5468):1013–9.
Duong HA, Robles MS, Knutti D, Weitz CJ. A molecular mechanism for circadian clock negative feedback. Science. 2011;332(6036):1436–9. doi:10.1126/science.1196766.
Levi F, Okyar A, Dulong S, Innominato PF, Clairambault J. Circadian timing in cancer treatments. Annu Rev Pharmacol Toxicol. 2010;50:377–421. doi:10.1146/annurev.pharmtox.48.113006.094626.
Zhang J, Dong X, Fujimoto Y, Okamura H. Molecular signals of Mammalian circadian clock. Kobe J Med Sci. 2004;50(3–4):101–9.
Sancar A, Lindsey-Boltz LA, Kang TH, Reardon JT, Lee JH, Ozturk N. Circadian clock control of the cellular response to DNA damage. FEBS Lett. 2010;584(12):2618–25. doi:10.1016/j.febslet.2010.03.017.
Rana S, Mahmood S. Circadian rhythm and its role in malignancy. J Circadian Rhythms.8:3. doi:10.1186/1740-3391-8-3.
Tokunaga H, Takebayashi Y, Utsunomiya H, Akahira J, Higashimoto M, Mashiko M, et al. Clinicopathological significance of circadian rhythm-related gene expression levels in patients with epithelial ovarian cancer. Acta Obstet Gynecol Scand. 2008;87(10):1060–70. doi:10.1080/00016340802348286.
Chen ST, Choo KB, Hou MF, Yeh KT, Kuo SJ, Chang JG. Deregulated expression of the PER1, PER2 and PER3 genes in breast cancers. Carcinogenesis. 2005;26(7):1241–6. doi:10.1093/carcin/bgi075.
Wood PA, Yang X, Taber A, Oh EY, Ansell C, Ayers SE, et al. Period 2 mutation accelerates ApcMin/+ tumorigenesis. Mol Cancer Res. 2008;6(11):1786–93. doi:10.1158/1541-7786.MCR-08-0196.
Oshima T, Takenoshita S, Akaike M, Kunisaki C, Fujii S, Nozaki A, et al. Expression of circadian genes correlates with liver metastasis and outcomes in colorectal cancer. Oncol Rep. 2011;25(5):1439–46. doi:10.3892/or.2011.1207.
Chen WQ, Zheng RS, Zhang SW. Liver cancer incidence and mortality in China. Cancer. 2009;32(4):162–9. doi:10.5732/cjc.013.10027.
Saika K, Sobue T. [Cancer statistics in the world]. Gan To Kagaku Ryoho. 2013;40(13):2475–80.
Laurent-Puig P, Zucman-Rossi J. Genetics of hepatocellular tumors. Oncogene. 2006;25(27):3778–86. doi:10.1038/sj.onc.1209547.
Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007;27(1):55–76. doi:10.1055/s-2006-960171.
Lin YM, Chang JH, Yeh KT, Yang MY, Liu TC, Lin SF, et al. Disturbance of circadian gene expression in hepatocellular carcinoma. Mol Carcinog. 2008;47(12):925–33. doi:10.1002/mc.20446.
Ulrich CM, Robien K, McLeod HL. Cancer pharmacogenetics: polymorphisms, pathways and beyond. Nat Rev Cancer. 2003;3(12):912–20. doi:10.1038/nrc1233.
Zhu Y, Stevens RG, Hoffman AE, Fitzgerald LM, Kwon EM, Ostrander EA, et al. Testing the circadian gene hypothesis in prostate cancer: a population-based case-control study. Cancer Res. 2009;69(24):9315–22. doi:10.1158/0008-5472.CAN-09-0648.
Zhao B, Lu J, Yin J, Liu H, Guo X, Yang Y, et al. A functional polymorphism in PER3 gene is associated with prognosis in hepatocellular carcinoma. Liver Int. 2012;32(9):1451–9. doi:10.1111/j.1478-3231.2012.02849.x.
Zhou F, He X, Liu H, Zhu Y, Jin T, Chen C, et al. Functional polymorphisms of circadian positive feedback regulation genes and clinical outcome of Chinese patients with resected colorectal cancer. Cancer. 2012;118(4):937–46. doi:10.1002/cncr.26348.
Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8(12):1065–6.
Winter SL, Bosnoyan-Collins L, Pinnaduwage D, Andrulis IL. Expression of the circadian clock genes Per1 and Per2 in sporadic and familial breast tumors. Neoplasia. 2007;9(10):797–800.
Yeh KT, Yang MY, Liu TC, Chen JC, Chan WL, Lin SF, et al. Abnormal expression of period 1 (PER1) in endometrial carcinoma. J Pathol. 2005;206(1):111–20. doi:10.1002/path.1756.
Pogue-Geile KL, Lyons-Weiler J, Whitcomb DC. Molecular overlap of fly circadian rhythms and human pancreatic cancer. Cancer Lett. 2006;243(1):55–7. doi:10.1016/j.canlet.2005.11.049.
Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell. 2006;22(3):375–82. doi:10.1016/j.molcel.2006.03.038.
Im JS, Jung BH, Kim SE, Lee KH, Lee JK. Per3, a circadian gene, is required for Chk2 activation in human cells. FEBS Lett. 2010;584(23):4731–4. doi:10.1016/j.febslet.2010.11.003.
Climent J, Perez-Losada J, Quigley DA, Kim IJ, Delrosario R, Jen KY, et al. Deletion of the PER3 gene on chromosome 1p36 in recurrent ER-positive breast cancer. J Clin Oncol. 2010;28(23):3770–8. doi:10.1200/JCO.2009.27.0215.
Ozturk N, Lee JH, Gaddameedhi S, Sancar A. Loss of cryptochrome reduces cancer risk in p53 mutant mice. Proc Natl Acad Sci USA. 2009;106(8):2841–6. doi:10.1073/pnas.0813028106.
Gauger MA, Sancar A. Cryptochrome, circadian cycle, cell cycle checkpoints, and cancer. Cancer Res. 2005;65(15):6828–34. doi:10.1158/0008-5472.CAN-05-1119.
Xu J, Shen ZY, Chen XG, Zhang Q, Bian HJ, Zhu P, et al. A randomized controlled trial of Licartin for preventing hepatoma recurrence after liver transplantation. Hepatology. 2007;45(2):269–76. doi:10.1002/hep.21465.
Zhu Y, Stevens RG, Leaderer D, Hoffman A, Holford T, Zhang Y, et al. Non-synonymous polymorphisms in the circadian gene NPAS2 and breast cancer risk. Breast Cancer Res Treat. 2008;107(3):421–5. doi:10.1007/s10549-007-9565-0.
Zhu Y, Leaderer D, Guss C, Brown HN, Zhang Y, Boyle P, et al. Ala394Thr polymorphism in the clock gene NPAS2: a circadian modifier for the risk of non-Hodgkin’s lymphoma. Int J Cancer. 2007;120(2):432–5. doi:10.1002/ijc.22321.
Shastry BS. SNPs: impact on gene function and phenotype. Methods Mol Biol. 2009;578:3–22. doi:10.1007/978-1-60327-411-1_1.
Hull J, Campino S, Rowlands K, Chan MS, Copley RR, Taylor MS, et al. Identification of common genetic variation that modulates alternative splicing. PLoS Genet. 2007;3(6):e99. doi:10.1371/journal.pgen.0030099.
Yamaguchi S, Shinmura K, Saitoh T, Takenoshita S, Kuwano H, Yokota J. A single nucleotide polymorphism at the splice donor site of the human MYH base excision repair genes results in reduced translation efficiency of its transcripts. Genes Cells. 2002;7(5):461–74.
Acknowledgments
This work was supported by Program for New Century Excellent Talents in University, grants 81201583 and 81171966 from the National Natural Science Foundation, grant S2011GR0239 from the International S&T Cooperation Program and grant 2011ZX09307-001-04 from National Key Scientific and Technological Project of China.
Conflict of interest
The authors have declared that no competing interests exist.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Zhaohui Zhang and Fei Ma have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, Z., Ma, F., Zhou, F. et al. Functional polymorphisms of circadian negative feedback regulation genes are associated with clinical outcome in hepatocellular carcinoma patients receiving radical resection. Med Oncol 31, 179 (2014). https://doi.org/10.1007/s12032-014-0179-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0179-1