Abstract
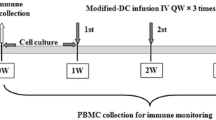
The aim of this study was to determine the efficacy and toxicity of pemetrexed plus dendritic cells (DCs) in patients suffering from stage IIIB or IV lung adenocarcinoma, who had undergone maintenance treatment with gefitinib or erlotinib. Patients who had failed gefitinib or erlotinib maintenance treatment had ECOG performance statuses ranging from 0 to 2.27 patients received pemetrexed plus DCs as second-line treatment. Dosage: 500 mg/m2 pemetrexed was administered on day 1 of a 21-day cycle. DCs were given for one cycle of 21 days. Three patients (11.1 %) experienced a partial response and 14 patients (51.9 %) showed stable disease. Ten patients (37.0 %) had progressive disease. The median time to progression-free survival (PFS) was 4.8 months [95 % confidence interval (CI) 4.4–5.2], and the median overall survival was 10.7 months (95 % CI 10.3–11.2). In the subgroup analysis, PFS had a significant difference between the low ratio of CD4/CD8 and normal ratio of CD4/CD8, with 4.5 months (95 % CI 4.2–4.9) and 5.0 months (95 % CI 4.5–5.7), (Log Rank = 0.039), respectively. No one patient experienced grade 4 toxicity. A regimen of pemetrexed combined with DCs is marginally effective and well tolerated in patients with stage IIIB or IV lung adenocarcinoma who had received gefitinib or erlotinib first-line treatment.



Similar content being viewed by others
References
NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26(28):4617–25.
Holmberg L, Sandin F, Bray F, Richards M, Spicer J, Lambe M, et al. National comparisons of lung cancer survival in England, Norway and Sweden 2001–2004: differences occur early in follow-up. Thorax. 2010;65:436–41.
Ozkaya S, Findik S, Atici AG, et al. Cisplatin-based chemotherapy in elderly patients with advanced stage (IIIB and IV) non-small cell lung cancer patients. Neoplasma. 2011;58(4):348–51.
Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374(9699):1432–40.
Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11(6):521–9.
Gaafar RM, Surmont VF, Scagliotti GV, et al. A double-blind, randomised, placebo-controlled phase III intergroup study of gefitinib in patients with advanced NSCLC, non-progressing after first line platinum-based chemotherapy (EORTC 08021/ILCP 01/03). Eur J Cancer. 2011;47(15):2331–40.
Schiller JH, von Pawel J, Schütt P, et al. Pemetrexed with or without matuzumab as second-line treatment for patients with stage IIIB/IV non-small cell lung cancer. J Thorac Oncol. 2010;5(12):1977–85.
Adjei AA, Mandrekar SJ, Dy GK, et al. Phase II trial of pemetrexed plus bevacizumab for second-line therapy of patients with advanced non-small-cell lung cancer: NCCTG and SWOG study N0426. J Clin Oncol. 2010;28(4):614–9.
Van den Heuvel MM, Burgers SA, van Zandwijk N. Immunotherapy in nonsmall-cell lung carcinoma: from inflammation to vaccination. Clin Lung Cancer. 2009;10:99–105.
Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–52.
Gunzer M, Jänich S, Varga G, Grabbe S. Dendritic cells and tumor immunity. Semin Immunol. 2001;13:291–302.
Whiteside TL, Odoux C. Dendritic cell biology and cancer therapy. Cancer Immunol Immunother. 2004;53:240–8.
Märten A, Greten T, Ziske C, Renoth S, Schöttker B, Buttgereit P, Schakowski F, von Rücker A, Sauerbruch T, Schmidt-Wolf IH. Generation of activated and antigen specific T cells with cytotoxic activity after co-culture with dendritic cells. Cancer Immunol Immunother. 2002;51:25–32.
Wilke CM, Kryczek I, Zou W. Antigen-presenting cell (APC) subsets in ovarian cancer. Int Rev Immunol. 2011;30:120–6.
Ganul AV, Khranovskaya NN, Sovenko VM, et al. Experience of using dendritic cell autovaccine in non-small-cell lung cancer patients. Clin Oncol. 2012;3:21–5.
Tuyaerts S. Dendritic cell therapy for oncology roundtable conference. J Immune Based Ther Vaccines. 2011;9:1–7.
Yannelli JR, Sturgill J, Foody T, et al. The large scale generation of dendritic cells for the immunization of patients with non-small cell lung cancer (NSCLC). Lung Cancer. 2005;47(3):337–50.
Um SJ, Choi YJ, Shin HJ, et al. Phase I study of autologous dendritic cell tumor vaccine in patients with non-small cell lung cancer. Lung Cancer. 2010;70(2):188–94.
Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. Cancer J Clin. 2005;55(1):10–30.
Custodio A, de Castro J. Strategies for maintenance therapy in advanced non-small cell lung cancer: current status, unanswered questions and future directions. Crit Rev Oncol Hematol. 2012;82(3):338–60.
Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97.
Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97.
Heist RS, Fidias P, Huberman M, et al. A phase II study of oxaliplatin, pemetrexed, and bevacizumab in previously treated advanced non-small cell lung cancer. J Thorac Oncol. 2008;3(10):1153–8.
Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two phase III studies. Oncologist. 2009;14:253–63.
Yang CH, Simms L, Park K, et al. Efficacy and safety of cisplatin/pemetrexed versus cisplatin/gemcitabine as first-line treatment in East Asian patients with advanced non-small cell lung cancer: results of an exploratory subgroup analysis of a phase III trial. J Thorac Oncol. 2010;5:688–95.
Zoll B, Lefterova P, Ebert O, Huhn D, Von Ruecker A, Schmidt-Wolf IG. Modulation of cell surface markers on NK-like T lymphocytes by using IL-2, IL-7 or IL-12 in vitro stimulation. Cytokine. 2000;12:1385–1390. PMID: 10975999.
Perroud MW Jr, Honma HN, Barbeiro AS, et al. Mature autologous dendritic cell vaccines in advanced non-small cell lung cancer: a phase I pilot study. J Exp Clin Cancer Res. 2011;30:65.
Hald SM, Bremnes RM, Al-Shibli K, et al. CD4/CD8 co-expression shows independent prognostic impact in resected non-small cell lung cancer patients treated with adjuvant radiotherapy. Lung Cancer. 2013;80(2):209–15.
Skachkova OV, Khranovska NM, Gorbach OI, et al. Immunological markers of anti-tumor dendritic cells vaccine efficiency in patients with non-small cell lung cancer. Exp Oncol. 2013;35(2):109–13.
Scagliotti GV, Kortsik C, Dark GG, et al. Pemetrexed combined with oxaliplatin or carboplatin as first-line treatment in advanced non-small cell lung cancer: a multicenter, randomized, phase II trial. Clin Cancer Res. 2005;11(2):690–6.
Mir O, Boudou-Rouquette P, Giroux J, et al. Pemetrexed, oxaliplatin and bevacizumab as first-line treatment in patients with stage IV non-small cell lung cancer. Lung Cancer. 2012;77(1):104–9.
Conflict of interest
No conflicts of interest exist for any of the authors of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Rong-Hang Hu and Sheng-Bin Shi have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hu, RH., Shi, SB., Qi, JL. et al. Pemetrexed plus dendritic cells as second-line treatment for patients with stage IIIB/IV non-small cell lung cancer who had treatment with TKI. Med Oncol 31, 63 (2014). https://doi.org/10.1007/s12032-014-0063-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0063-z