Abstract
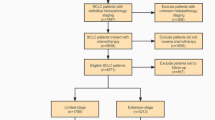
A clinical prognostic model derived from BR.21 trial was established by Florescu et al., which helped to identify a small group of patients with non-small cell lung cancer (NSCLC) who might be less likely to benefit from erlotinib therapy. Whether the prognostic model derived from Caucasian patients treated with erlotinib will be applied to Asian patients treated with gefitinib is still an open question. We reviewed a multi-center clinical trial of Chinese patients with NSCLC treated with gefitinib. The data were collected and analyzed according to the prognostic model reported by Florescu et al. One hundred and nineteen patients were included in the validation study. Twenty-eight patients, 61 patients, 27 patients, and 3 patients were classified into the Low Risk (LR) group, Intermediate Low Risk (ILR) group, Intermediate High Risk/High Risk (IHR/HR) group, respectively. The median overall survival of LR group was not reached, ILR and IHR/HR group was 8.9 months and 4.5 months, respectively. There was a significant difference in overall survival between LR group versus ILR group and IHR/HR group (P = 0.0003 and 0.0001, respectively). While IHR/HR group appeared to have less survival benefit than ILR group, the difference was not statistically significant (P = 0.148). The result has shown a similar effect as that seen by Florescu et al. in differentiating patient risk groups. Our study provides the potential evidence that the prognostic model might be applied to Asian patients with NSCLC treated with gefitinib and helps clinicians to select patients for gefitinib therapy and stratify patients within second-line clinical trials.

Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, et al. Cancer statistics, 2007. Cancer J Clin. 2007;57:43–66.
Baggstrom MQ, Stinchcombe TE, Fried DB, Poole C, Hensing TA, et al. Third-generation chemotherapy agents in the treatment of advanced non-small cell lung cancer: a meta-analysis. J Thorac Oncol. 2007;2:845–53.
Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103.
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32.
Thatcher N, Chang A, Parikh P, Pereira JR, Ciuleanu T. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small cell lung cancer: results from a randomized, placebocontrolled, multicentre study (Iressa Survival Evaluation in Lung Cancer, ISEL study). Lancet. 2005;366:1527–37.
Clark GM, Zborowski DM, Santabarbara P, Ding K, Whitehead M, et al. Smoking history and epidermal growth factor receptor expression as predictors of survival benefit from erlotinib for patients with non-small-cell lung cancer in the National Cancer Institute of Canada Clinical Trials Group study BR.21. Clin Lung Cancer. 2006;7:389–94.
Cufer T, Vrdoljak E, Gaafar R, Erensoy I, Pemberton K. SIGN Study Group. Phase II, open-label, randomized study (SIGN) of single-agent gefitinib (IRESSA) or docetaxel as second-line therapy in patients with advanced (stage IIIb or IV) non-small-cell lung cancer. Anticancer Drugs. 2006;17:401–9.
Maruyama R, Nishiwaki Y, Tamura T, Yamamoto N, Tsuboi M, et al. Phase III study, V-15–32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol. 2008;26:4244–52.
Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372:1809–18.
Florescu M, Hasan B, Seymour L, Ding K, Shepherd FA. National Cancer Institute of Canada Clinical Trials Group. A clinical prognostic index for patients treated with erlotinib in National Cancer Institute of Canada Clinical Trials Group study BR.21. J Thorac Oncol. 2008;3:590–8.
Guan ZZ, Zhang L, Li LY, Jiang GL, Liu XY, et al. Efficacy of gefitinib on Chinese patients with locally advanced or metastatic non-small cell lung cancer: a clinical trial. Ai Zheng. 2005;24:980–4. Article in Chinese.
Park MJ, Lee J, Hong JY, Choi MK, Yi JH, et al. Prognostic model to predict outcomes in nonsmall cell lung cancer patients treated with gefitinib as a salvage treatment. Cancer. 2009;115:1518–30.
Satouchi M, Negoro S, Funada Y, Urata Y, Shimada T, et al. Predictive factors associated with prolonged survival in patients with advanced non-small-cell lung cancer (NSCLC) treated with gefitinib. Br J Cancer. 2007;96:1191–6.
Han SW, Kim TY, Hwang PG, Jeong S, Kim J, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2005;23:2493–501.
Mitsudomi T, Kosaka T, Endoh H, Horio Y, Hida T, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small cell lung cancer with postoperative recurrence. J Clin Oncol. 2005;23:2513–20.
Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005;97:643–55.
Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with nonsmall- cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–9.
Massarelli E, Varella-Garcia M, Tang X, Xavier AC, Ozburn NC, et al. KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin Cancer Res. 2007;13:2890–6.
Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, et al. Gefitinib or Carboplatin-Paclitaxel in Pulmonary Adenocarcinoma. N Engl J Med. 2009;361:947–57.
Author information
Authors and Affiliations
Corresponding author
Additional information
Fenghua Wang, Yang Zhang authors contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Wang, F., Zhang, Y., Zhao, H. et al. Validation of a clinical prognostic model in Chinese patients with metastatic and advanced pretreated non-small cell lung cancer treated with gefitinib. Med Oncol 28, 331–335 (2011). https://doi.org/10.1007/s12032-010-9451-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9451-1