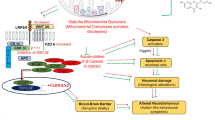
Abstract
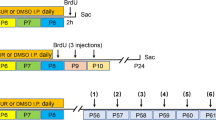
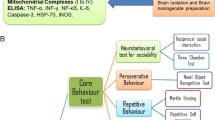
Autism is a neurodevelopmental condition, and it's associated pathophysiology, viz., oxidative stress and altered cellular homeostasis, has been extensively intertwined with behavioral impairments. Therefore, targeting oxidative stress and redox cellular homeostasis could be beneficial in relieving autistic-like symptoms. For this purpose, we examined a library of nutraceutical compounds that led us to a bioflavonoid fisetin. Autism-like neurobehavior was induced by subjecting the pregnant rodents to valproic acid at the time of neural tube closure (GD12.5). In this novel study, fisetin was evaluated for its neuroprotective potential at gestational (GD13 until delivery) and post-weaning developmental windows (PND 23–32) in VPA-induced rodent model of autism. Developmental VPA exposure increased intracellular ROS production, oxidative stress, altered AChE and ATPases in brain regions, and induced autistic-like behavioral impairments (social, repetitive, stereotyped, and sensorimotor). The present findings suggested that gestational and post-weaning fisetin treatment significantly improved the behavioral impairments by attenuating elevated oxidative stress, ROS, lipid peroxidation, and re-establishing redox homeostasis. Also, it effectively reinstated the reduced levels of endogenous antioxidants, glutathione, AChE, and ATPases by its antioxidant potential. Therefore, fisetin with its properties could be used as a potential therapeutic agent in overcoming the symptoms associated with autism.
Graphical Abstract








Similar content being viewed by others
Availability Data and Materials
All data generated or analyzed during this study are included in this manuscript and its supplementary information files.
References
Ádám Á, Kemecsei R, Company V, Murcia-Ramón R, Juarez I, Gerecsei LI et al (2020) Gestational exposure to sodium valproate disrupts fasciculation of the mesotelencephalic dopaminergic tract, with a selective reduction of dopaminergic output from the ventral tegmental area. Front Neuroanat 14:29. https://doi.org/10.3389/fnana.2020.00029. PMID32581730
Ahmad SF, Nadeem A, Ansari MA, Bakheet SA, Al-Ayadhi LY, Attia SM (2017) Upregulation of IL-9 and JAK-STAT signaling pathway in children with autism. Prog Neuropsychopharmacol Biol Psychiatry 79(B):472–480. https://doi.org/10.1016/j.pnpbp.2017.08.002. PMID 28802860
Ahsan AU, Sharma VL, Wani A, Chopra M (2020) Naringenin upregulates AMPK-mediated autophagy to rescue neuronal cells from β-amyloid (1–42) evoked neurotoxicity. Mol Neurobiol 57(8):3589–3602. https://doi.org/10.1007/s12035-020-01969-4. PMID32542594
Ajayi AF, Akhigbe RE (2020) Staging of the estrous cycle and induction of estrus in experimental rodents: an update. Fertil Res Pract 6:5. https://doi.org/10.1186/s40738-020-00074-3. PMID32190339
Al-Askar M, Bhat RS, Selim M, Al-Ayadhi L, El-Ansary A (2017) Postnatal treatment using curcumin supplements to amend the damage in VPA-induced rodent models of autism. BMC Complement Altern Med 17(1):259. https://doi.org/10.1186/s12906-017-1763-7. PMID28486989
Al-Gadani Y, El-Ansary A, Attas O, Al-Ayadhi L (2009) Metabolic biomarkers related to oxidative stress and antioxidant status in Saudi autistic children. Clin Biochem 42(10–11):1032–1040. https://doi.org/10.1016/j.clinbiochem.2009.03.011. PMID19306862
Alvarez-Arellano L, Salazar-García M, Corona JC (2020) Neuroprotective effects of quercetin in pediatric neurological diseases. Molecules 25(23):5597. https://doi.org/10.3390/molecules25235597. PMID33260783
Alwan S (2015) Chambers CD (2015) Identifying human teratogens: an update. J Pediatr Genet 4(2):39–41. https://doi.org/10.1055/s-0035-1556745. PMID27617116
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Publishing, Arlington
Baek BS, Kwon HJ, Lee KH, Yoo MA, Kim KW, Ikeno Y et al (1999) Regional difference of ROS generation, lipid peroxidation, and antioxidant enzyme activity in rat brain and their dietary modulation. Arch Pharm Res 22(4):361–366. https://doi.org/10.1007/BF02979058. PMID10489874
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–8. PMID13967893
Bolotta A, Visconti P, Fedrizzi G, Ghezzo A, Marini M, Manunta P et al (2018) Na+, K+ -ATPase activity in children with autism spectrum disorder: searching for the reason(s) of its decrease in blood cells. Autism Res 11(10):1388–1403. https://doi.org/10.1002/aur.2002. PMID30120881
Bonting SL, Simon KA, Hawkins NM (1961) Studies on sodium-potassium-activated adenosine triphosphatase. I. Quantitative distribution in several tissues of the cat. Arch Biochem Biophys 95:416–423. https://doi.org/10.1016/0003-9861(61)90170-9. PMID13871109
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310. https://doi.org/10.1016/s0076-6879(78)52032-6. PMID672633
Cezar LC, Kirsten TB, da Fonseca CCN, de Lima APN, Bernardi MM, Felicio LF (2018) Zinc as a therapy in a rat model of autism prenatally induced by valproic acid. Prog Neuropsychopharmacol Biol Psychiatry 84(A):173–180. https://doi.org/10.1016/j.pnpbp.2018.02.008. PMID29481896
Chahoud I, Paumgartten FJ (2009) Influence of litter size on the postnatal growth of rat pups: is there a rationale for litter-size standardization in toxicity studies? Environ Res 109(8):1021–1027. https://doi.org/10.1016/j.envres.2009.07.015. PMID19762015
Chaste P, Leboyer M (2012) Autism risk factors: genes, environment, and gene-environment interactions. Dialogues Clin Neurosci 14(3):281–292. https://doi.org/10.31887/DCNS.2012.14.3/pchaste. PMID23226953
Chen R, Lai UH, Zhu L, Singh A, Ahmed M, Forsyth NR (2018) Reactive oxygen species formation in the brain at different oxygen levels: the role of hypoxia inducible factors. Front Cell Dev Biol 6:132. https://doi.org/10.3389/fcell.2018.00132. PMID30364203
Chen X, Yue J, Luo Y, Huang L, Li B, Wen S (2021) Distinct behavioral traits and associated brain regions in mouse models for obsessive-compulsive disorder. Behav Brain Funct 17(1):4. https://doi.org/10.1186/s12993-021-00177-x. PMID34006308
Chen Y, Qin C, Huang J, Tang X, Liu C, Huang K et al (2020) The role of astrocytes in oxidative stress of central nervous system: a mixed blessing. Cell Prolif 53(3):e12781. https://doi.org/10.1111/cpr.12781. PMID32035016.
Cheng N, Rho JM, Masino SA (2017) Metabolic dysfunction underlying autism spectrum disorder and potential treatment approaches. Front Mol Neurosci 10:34. https://doi.org/10.3389/fnmol.2017.00034. PMID28270747
Clement JG (1994) Chromodacryorrhea in rats: absence following soman poisoning. Toxicol Appl Pharmacol 124(1):52–58. https://doi.org/10.1006/taap.1994.1007. PMID: 8291061
de Mattos BDS, Soares MSP, Spohr L, Pedra NS, Teixeira FC, de Souza AA et al (2020) Quercetin prevents alterations of behavioral parameters, delta-aminolevulinic dehydratase activity, and oxidative damage in brain of rats in a prenatal model of autism. Int J Dev Neurosci 80(4):287–302. https://doi.org/10.1002/jdn.10025. PMID32181519
Dufour-Rainfray D, Vourc’h P, Tourlet S, Guilloteau D, Chalon S, Andres CR (2011) Fetal exposure to teratogens: evidence of genes involved in autism. Neurosci Biobehav Rev 35(5):1254–1265. https://doi.org/10.1016/j.neubiorev.2010.12.013. PMID21195109
Eissa N, Azimullah S, Jayaprakash P, Jayaraj RL, Reiner D, Ojha SK et al (2019) The dual-active histamine H3 receptor antagonist and acetylcholine esterase inhibitor E100 ameliorates stereotyped repetitive behavior and neuroinflammmation in sodium valproate induced autism in mice. Chem Biol Interact 312:108775. https://doi.org/10.1016/j.cbi.2019.108775. PMID31369746.
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcín C et al (2012) Global prevalence of autism and other pervasive developmental disorders. Autism Res 5(3):160–179. https://doi.org/10.1002/aur.239. PMID22495912
Favre MR, Barkat TR, Lamendola D, Khazen G, Markram H, Markram K (2013) General developmental health in the VPA-rat model of autism. Front Behav Neurosci 7:88. https://doi.org/10.3389/fnbeh.2013.00088. PMID23898245
Fereshetyan K, Chavushyan V, Danielyan M, Yenkoyan K (2021) Assessment of behavioral, morphological and electrophysiological changes in prenatal and postnatal valproate induced rat models of autism spectrum disorder. Sci Rep 11(1):23471. https://doi.org/10.1038/s41598-021-02994-6. PMID34873263
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. Biol Chem 66(2):375–400. https://doi.org/10.1016/S0021-9258(18)84756-1
Fontes-Dutra M, Santos-Terra J, Deckmann I, Brum Schwingel G, Della-Flora Nunes G, Hirsch MM et al (2018) Resveratrol prevents cellular and behavioral sensory alterations in the animal model of autism induced by valproic acid. Front Synaptic Neurosci 10:9. https://doi.org/10.3389/fnsyn.2018.00009. PMID29872390
Frye RE, Cakir J, Rose S, Palmer RF, Austin C, Curtin P et al (2021) Mitochondria may mediate prenatal environmental influences in autism spectrum disorder. J Pers Med 11(3):218. https://doi.org/10.3390/jpm11030218. PMID33803789
Gąssowska-Dobrowolska M, Cieślik M, Czapski GA, Jęśko H, Frontczak-Baniewicz M, Gewartowska M et al (2020) Prenatal exposure to valproic acid affects microglia and synaptic ultrastructure in a brain-region-specific manner in young-adult male rats: relevance to autism spectrum disorders. Int J Mol Sci 21(10):3576. https://doi.org/10.3390/ijms21103576. PMID32443651
Goel HC, Sajikumar S, Sharma A (2002) Effects of podophyllum hexandrum on radiation induced delay of postnatal appearance of reflexes and physiological markers in rats irradiated in utero. Phytomedicine 9(5):447–454. https://doi.org/10.1078/09447110260571715. PMID12222667
Griffiths KK, Levy RJ (2017) Evidence of mitochondrial dysfunction in autism: biochemical links, genetic-based associations, and non-energy-related mechanisms. Oxid Med Cell Longev 2017:4314025. https://doi.org/10.1155/2017/4314025. PMID28630658
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–9. https://doi.org/10.1016/S0021-9258(19)42083-8. PMID4436300.
Hassan SSU, Samanta S, Dash R, Karpiński TM, Habibi E, Sadiq A et al (2022) The neuroprotective effects of fisetin, a natural flavonoid in neurodegenerative diseases: focus on the role of oxidative stress. Front Pharmacol 13:1015835. https://doi.org/10.3389/fphar.2022.1015835. PMID36299900
He L, He T, Farrar S, Ji L, Liu T, Ma X (2017) Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem 44(2):532–553. https://doi.org/10.1159/000485089. PMID29145191
Hjertén S, Pan H (1983) Purification and characterization of two forms of a low-affinity Ca2+-ATPase from erythrocyte membranes. Biochim Biophys Acta 728(2):281–288. https://doi.org/10.1016/0005-2736(83)90480-7. PMID: 6219703
Hodges H, Fealko C, Soares N (2020) Autism spectrum disorder: definition, epidemiology, causes, and clinical evaluation. Transl Pediatr 9(Suppl 1):S55–S65. https://doi.org/10.21037/tp.2019.09.09. PMID32206584
Honarmand Tamizkar K, Badrlou E, Aslani T, Brand S, Arsang-Jang S, Ghafouri-Fard S et al (2021) Dysregulation of NF-κB-associated LncRNAs in autism spectrum disorder. Front Mol Neurosci 14:747785. https://doi.org/10.3389/fnmol.2021.747785. PMID34658787.
Horiquini Barbosa E, Vallim JH, Lachat JJ, de Castro VL (2016) Assessments of motor abnormalities on the grid-walking and foot-fault tests from undernutrition in Wistar rats. J Mot Behav 48(1):5–12. https://doi.org/10.1080/00222895.2015.1024824. PMID25923475
Jacob S, Thangarajan S (2017) Effect of gestational intake of fisetin (3,3′,4′,7-Tetrahydroxyflavone) on developmental methyl mercury neurotoxicity in F1 generation rats. Biol Trace Elem Res 177(2):297–315. https://doi.org/10.1007/s12011-016-0886-x. PMID27815688
Ji L, Chauhan A, Brown WT, Chauhan V (2009) Increased activities of Na+/K+-ATPase and Ca2+/Mg2+-ATPase in the frontal cortex and cerebellum of autistic individuals. Life Sci 85(23–26):788–793. https://doi.org/10.1016/j.lfs.2009.10.008. PMID19863947
Kanner L (1943) Autistic disturbances of affective contact. Nerv Child 2:217–250
Karvat G, Kimchi T (2014) Acetylcholine elevation relieves cognitive rigidity and social deficiency in a mouse model of autism. Neuropsychopharmacology 39(4):831–840. https://doi.org/10.1038/npp.2013.274. PMID24096295
Kaushal S, Ahsan AU, Sharma VL, Chopra M (2019) Epigallocatechin gallate attenuates arsenic induced genotoxicity via regulation of oxidative stress in BALB/c mice. Mol Biol Rep 46(5):5355–5369. https://doi.org/10.1007/s11033-019-04991-5. PMID31350662
Kazdoba TM, Leach PT, Yang M, Silverman JL, Solomon M, Crawley JN (2016) Translational mouse models of autism: advancing toward pharmacological therapeutics. Curr Top Behav Neurosci 28:1–52. https://doi.org/10.1007/7854_2015_5003. PMID27305922
Khan N, Syed DN, Ahmad N, Mukhtar H (2013) Fisetin: a dietary antioxidant for health promotion. Antioxid Redox Signal 19(2):151–162. https://doi.org/10.1089/ars.2012.4901. PMID23121441
Khatoon S, Agarwal NB, Samim M, Alam O (2021) Neuroprotective effect of fisetin through suppression of IL-1R/TLR axis and apoptosis in pentylenetetrazole-induced kindling in mice. Front Neurol 12:689069. https://doi.org/10.3389/fneur.2021.689069, PMID 34354662
Khera R, Mehan S, Kumar S, Sethi P, Bhalla S, Prajapati A (2022) Role of JAK-STAT and PPAR-gamma signalling modulators in the prevention of autism and neurological dysfunctions. Mol Neurobiol 59(6):3888–3912. https://doi.org/10.1007/s12035-022-02819-1. PMID35437700
Kim KC, Kim P, Go HS, Choi CS, Yang SI, Cheong JH et al (2011) The critical period of valproate exposure to induce autistic symptoms in Sprague-Dawley rats. Toxicol Lett 201(2):137–142. https://doi.org/10.1016/j.toxlet.2010.12.018. PMID21195144
Kono Y (1978) Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 186(1):189–195. https://doi.org/10.1016/0003-9861(78)90479-4. PMID24422
Koren G, Nava-Ocampo (2006) AA, Moretti ME, Sussman R, Nulman I. Major malformations with valproic acid. Can Fam Physician 52(4):441–447. PMID16639967.
Kudlak M, Tadi P (2021) Physiology, muscarinic receptor. InStatPearls [Internet]. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK555909/
Kumar S, Reynolds K, Ji Y, Gu R, Rai S, Zhou CJ (2019) Impaired neurodevelopmental pathways in autism spectrum disorder: a review of signaling mechanisms and crosstalk. J Neurodev Disord 11(1):10. https://doi.org/10.1186/s11689-019-9268-y. PMID31202261
Kumaravel P, Melchias G, Vasanth N, Manivasagam T (2017) Epigallocatechin gallate attenuates behavioral defects in sodium valproate induced autism rat model. Res J Pharm Technol 10(5):1477–1480
Landolfo S, Politi H, Angelozzi D, Mannazzu I (2008) ROS accumulation and oxidative damage to cell structures in Saccharomyces cerevisiae wine strains during fermentation of high-sugar-containing medium. Biochim Biophys Acta 1780(6):892–898. https://doi.org/10.1016/j.bbagen.2008.03.008. PMID18395524
László K, Kiss O, Vörös D, Mintál K, Ollmann T, Péczely L et al (2022) Intraamygdaloid oxytocin reduces anxiety in the valproate-induced autism rat model. Biomedicines 10(2):405. https://doi.org/10.3390/biomedicines10020405. PMID35203614
Lázaro MT, Golshani P (2015) The utility of rodent models of autism spectrum disorders. Curr Opin Neurol 28(2):103–109. https://doi.org/10.1097/WCO.0000000000000183. PMID25734952
Lee KH, Cha M, Lee BH (2021) Crosstalk between neuron and glial cells in oxidative injury and neuroprotection. Int J Mol Sci 22(24):13315. https://doi.org/10.3390/ijms222413315. PMID34948108
Liu X, Lin J, Zhang H, Khan NU, Zhang J, Tang X et al (2022) Oxidative stress in autism spectrum disorder-current progress of mechanisms and biomarkers. Front Psychiatry 13:813304. https://doi.org/10.3389/fpsyt.2022.813304. PMID35299821.
Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J (2018) Autism spectrum disorder. Lancet 392(10146):508–520. https://doi.org/10.1016/S0140-6736(18)31129-2
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275. https://doi.org/10.1016/S0021-9258(19)52451-6. PMID14907713
Luck H (1971) Catalase. In: Hu B (ed) Methods of enzymatic analysis, vol 3, p 279
Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK et al (2017) The changing epidemiology of autism spectrum disorders. Annu Rev Public Health 38:81–102. https://doi.org/10.1146/annurev-publhealth-031816-044318. PMID28068486
Mabunga DF, Gonzales EL, Kim JW, Kim KC, Shin CY (2015) Exploring the validity of valproic acid animal model of autism. Exp Neurobiol 24(4):285–300. https://doi.org/10.5607/en.2015.24.4.285. PMID26713077
Maher P (2021) Preventing and treating neurological disorders with the flavonol fisetin. Brain Plast 6(2):155–166. https://doi.org/10.3233/BPL-200104. PMID33782648
Massaad CA, Klann E (2011) Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid Redox Signal 14(10):2013–2054. https://doi.org/10.1089/ars.2010.3208. PMID20649473
Maurissen JP, Hoberman AM, Garman RH, Hanley TR Jr (2000) Lack of selective developmental neurotoxicity in rat pups from dams treated by gavage with chlorpyrifos. Toxicol Sci 57(2):250–263. https://doi.org/10.1093/toxsci/57.2.250. PMID11006355
Mehra S, Ul Ahsan A, Seth E, Chopra M (2022) Critical evaluation of valproic acid-induced rodent models of autism: current and future perspectives. J Mol Neurosci 72(6):1259–1273. https://doi.org/10.1007/s12031-022-02033-7. PMID35635674
Modabbernia A, Velthorst E, Reichenberg A (2017) Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol Autism 8:13. https://doi.org/10.1186/s13229-017-0121-4. PMID28331572
Morakotsriwan N, Wattanathorn J, Kirisattayakul W, Chaisiwamongkol K (2016) Autistic-like behaviors, oxidative stress status, and histopathological changes in cerebellum of valproic acid rat model of autism are improved by the combined extract of purple rice and silkworm pupae. Oxid Med Cell Longev 2016:3206561. https://doi.org/10.1155/2016/3206561. PMID27034733
Mousain-Bosc M, Siatka C, Bali JP (2011) Magnesium, hyperactivity and autism in children. In: Vink R, Nechifor M, editors. Magnesium in the central nervous system [Internet]. Adelaide (AU): University of Adelaide Press. PMID: 29920003.
Muckova L, Vanova N, Misik J, Herman D, Pejchal J, Jun D (2019) Oxidative stress induced by oxime reactivators of acetylcholinesterase in vitro. Toxicol in Vitro 56:110–117. https://doi.org/10.1016/j.tiv.2019.01.013. PMID30682493
Mychasiuk R, Richards S, Nakahashi A, Kolb B, Gibb R (2012) Effects of rat prenatal exposure to valproic acid on behaviour and neuro-anatomy. Dev Neurosci 34(2–3):268–276. https://doi.org/10.1159/000341786. PMID22890088
Nabavi SF, Braidy N, Habtemariam S, Sureda A, Manayi A, Nabavi SM (2016) Neuroprotective effects of fisetin in Alzheimer’s and Parkinson’s diseases: from chemistry to medicine. Curr Top Med Chem 16(17):1910–1915. https://doi.org/10.2174/1568026616666160204121725. PMID26845554
Napoli E, Wong S, Giulivi C (2013) Evidence of reactive oxygen species-mediated damage to mitochondrial DNA in children with typical autism. Mol Autism 4(1):2. https://doi.org/10.1186/2040-2392-4-2. PMID23347615
Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R (2008) Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol 153(1):6–20. https://doi.org/10.1038/sj.bjp.0707395. PMID17643134.
Nicolini C, Fahnestock M (2018) The valproic acid-induced rodent model of autism. Exp Neurol. 2018;299(A):217–227. https://doi.org/10.1016/j.expneurol.2017.04.017. PMID28472621.
Ohnishi T, Suzuki T, Suzuki Y, Ozawa K (1982) A comparative study of plasma membrane Mg2+-ATPase activities in normal, regenerating and malignant cells. Biochim Biophys Acta 684(1):67–74. https://doi.org/10.1016/0005-2736(82)90050-5
Ornoy A, Weinstein-Fudim L, Ergaz Z (2016) Genetic syndromes, maternal diseases and antenatal factors associated with autism spectrum disorders (ASD). Front Neurosci 10:316. https://doi.org/10.3389/fnins.2016.00316. PMID27458336
Oswald MCW, Garnham N, Sweeney ST, Landgraf M (2018) Regulation of neuronal development and function by ROS. FEBS Lett 592(5):679–691. https://doi.org/10.1002/1873-3468.12972. PMID29323696
Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: an overview. J Nutr Sci 5:e47. https://doi.org/10.1017/jns.2016.41. PMID28620474.
Pangrazzi L, Balasco L, Bozzi Y (2020) Oxidative stress and immune system dysfunction in autism spectrum disorders. Int J Mol Sci 21(9):3293. https://doi.org/10.3390/ijms21093293. PMID32384730
Paris JJ, Brunton PJ, Russell JA, Frye CA (2011) Immune stress in late pregnant rats decreases length of gestation and fecundity, and alters later cognitive and affective behaviour of surviving pre-adolescent offspring. Stress (amsterdam Neth) 14(6):652–664. https://doi.org/10.3109/10253890.2011.628719. PMID21995525
Pathak N, Khandelwal S (2009) Immunomodulatory role of piperine in cadmium induced thymic atrophy and splenomegaly in mice. Environ Toxicol Pharmacol 28(1):52–60. https://doi.org/10.1016/j.etap.2009.02.003. PMID21783982
Pragnya B, Kameshwari JS, Veeresh B (2014) Ameliorating effect of piperine on behavioral abnormalities and oxidative markers in sodium valproate induced autism in BALB/c mice. Behav Brain Res 270:86–94. https://doi.org/10.1016/j.bbr.2014.04.045. PMID24803211
Qiu J, Singh P, Pan G, de Paolis A, Champagne FA, Liu J et al (2020) Defining the relationship between maternal care behavior and sensory development in Wistar rats: auditory periphery development, eye opening and brain gene expression. PLOS ONE 15(8):e0237933. https://doi.org/10.1371/journal.pone.0237933. PMID32822407.
Randolph-Gips M, Srinivasan P (2012) Modeling autism: a systems biology approach. J Clin Bioinforma 2(1):17. https://doi.org/10.1186/2043-9113-2-17. PMID23043674
Rasalam AD, Hailey H, Williams JH, Moore SJ, Turnpenny PD, Lloyd DJ et al (2005) Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev Med Child Neurol 47(8):551–555. https://doi.org/10.1017/s0012162205001076. PMID16108456
Ravula AR, Teegala SB, Kalakotla S, Pasangulapati JP, Perumal V, Boyina HK (2021) Fisetin, potential flavonoid with multifarious targets for treating neurological disorders: an updated review. Eur J Pharmacol 910:174492. https://doi.org/10.1016/j.ejphar.2021.174492. PMID34516952.
Rosina E, Battan B, Siracusano M, Di Criscio L, Hollis F, Pacini L et al (2019) Disruption of mTOR and MAPK pathways correlates with severity in idiopathic autism. Transl Psychiatry 9(1):50. https://doi.org/10.1038/s41398-018-0335-z. PMID30705255
Rossignol DA, Frye RE (2014) Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front Physiol 5:150. https://doi.org/10.3389/fphys.2014.00150. PMID24795645
Roullet FI, Lai JK, Foster JA (2013) In utero exposure to valproic acid and autism–a current review of clinical and animal studies. Neurotoxicol Teratol 36:47–56. https://doi.org/10.1016/j.ntt.2013.01.004. PMID23395807
Ruhela RK, Soni S, Sarma P, Prakash A, Medhi B (2019) Negative geotaxis: an early age behavioral hallmark to VPA rat model of autism. Ann Neurosci 26(1):25–31. https://doi.org/10.5214/ans.0972.7531.260106. PMID31975769
Salim S (2017) Oxidative stress and the central nervous system. J Pharmacol Exp Ther 360(1):201–205. https://doi.org/10.1124/jpet.116.237503. PMID27754930
Schneider T, Labuz D, Przewłocki R (2001) Nociceptive changes in rats after prenatal exposure to valproic acid. Pol J Pharmacol 53(5):531–534. PMID11990073
Schneider T, Przewłocki R (2005) Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology 30(1):80–89. https://doi.org/10.1038/sj.npp.1300518. PMID: 15238991
Schrier MS, Zhang Y, Trivedi MS, Deth RC (2022) Decreased cortical Nrf2 gene expression in autism and its relationship to thiol and cobalamin status. Biochimie 192:1–12. https://doi.org/10.1016/j.biochi.2021.09.006. PMID34517051
Seth E, Ahsan AU, Kaushal S, Mehra S, Chopra M (2021) Berberine affords protection against oxidative stress and apoptotic damage in F1 generation of Wistar rats following lactational exposure to chlorpyrifos. Pestic Biochem Physiol 179:104977. https://doi.org/10.1016/j.pestbp.2021.104977. PMID34802527
Smith V, Brown N (2014) Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. Arch Dis Child Educ Pract Ed 99(5):198. https://doi.org/10.1136/archdischild-2013-305636. PMID24692263
Snezhkina AV, Kudryavtseva AV, Kardymon OL, Savvateeva MV, Melnikova NV, Krasnov GS et al (2019) ROS Generation and antioxidant defense systems in normal and malignant cells. Oxid Med Cell Longev 2019:6175804. https://doi.org/10.1155/2019/6175804. PMID31467634
Sultana R, Perluigi M, Butterfield DA (2013) Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic Biol Med 62:157–169. https://doi.org/10.1016/j.freeradbiomed.2012.09.027. PMID23044265
Taylor MJ, Rosenqvist MA, Larsson H, Gillberg C, D’Onofrio BM, Lichtenstein P et al (2020) Etiology of autism spectrum disorders and autistic traits over time. JAMA Psychiat 77(9):936–943. https://doi.org/10.1001/jamapsychiatry.2020.0680. PMID32374377
Terzi A, Alam SMS, Suter DM (2021) ROS Live Cell imaging during neuronal development. J Vis Exp 168(168). https://doi.org/10.3791/62165. PMID33645566
Terzioğlu Bebitoğlu B, Oğuz E, Gökçe A (2020) Effect of valproic acid on oxidative stress parameters of glutamate-induced excitotoxicity in SH-SY5Y cells. Exp Ther Med 20(2):1321–1328. https://doi.org/10.3892/etm.2020.8802. PMID32742366
Vithayathil J, Pucilowska J, Landreth GE (2018) ERK/MAPK signaling and autism spectrum disorders. Prog Brain Res 241:63–112. https://doi.org/10.1016/bs.pbr.2018.09.008. PMID30447757
Wilde M, Constantin L, Thorne PR, Montgomery JM, Scott EK, Cheyne JE (2022) Auditory processing in rodent models of autism: a systematic review. J Neurodev Disord 14(1):48. https://doi.org/10.1186/s11689-022-09458-6. PMID36042393
Yang M, Silverman JL, Crawley JN (2011) Automated three-chambered social approach task for mice. Curr Protoc Neurosci Chapter 8:Unit 8.26. https://doi.org/10.1002/0471142301.ns0826s56. PMID21732314
Yang EJ, Ahn S, Lee K, Mahmood U, Kim HS (2016) Early behavioral abnormalities and perinatal alterations of PTEN/AKT pathway in valproic acid autism model mice. PLoS One 11(4):e0153298. https://doi.org/10.1371/journal.pone.0153298. PMID27071011
Žigman T, Petković Ramadža D, Šimić G, Barić I (2021) Inborn errors of metabolism associated with autism spectrum disorders: approaches to intervention. Front Neurosci 15:673600. https://doi.org/10.3389/fnins.2021.673600. PMID34121999
Zunec S, Kopjar N, Zeljezić D, Kuca K, Musilek K, Lucić Vrdoljak A (2014) In vivo evaluation of cholinesterase activity, oxidative stress markers, cyto- and genotoxicity of K048 oxime–a promising antidote against organophosphate poisoning. Basic Clin Pharmacol Toxicol 114(4):344–351. https://doi.org/10.1111/bcpt.12158. PMID24741714
Acknowledgements
We are thankful to the Council of Scientific and Industrial Research (CSIR) for providing financial assistance to Ms. Sweety Mehra to carry out the research work. A vote of thanks goes to the Department of Zoology, Panjab University, Chandigarh, where the above-mentioned experimental work was carried out. Special thanks to Dr. Surbhi Kaushal and Dr. Naveed Pervaiz for their constant support during the research work.
Funding
Ms. Sweety Mehra received CSIR Research fellowship to carry out the mentioned work (09/135/2017 (790)-EMR-I).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Sweety Mehra, Aitizaz Ul Ahsan, Madhu Sharma, Muskan Budhwar, and Mani Chopra. The first draft of the manuscript (along with figures) was prepared by Sweety Mehra, and all the authors commented on all versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
The study was approved by the Institutional Animal Ethics Committee (Approval No: (PU/45/99/CPCSEA/IAEC/2019/271). All experiments were conducted in compliance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Consent to Participate
N/A.
Consent for Publication
N/A.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mehra, S., Ahsan, A.U., Sharma, M. et al. Neuroprotective Efficacy of Fisetin Against VPA-Induced Autistic Neurobehavioral Alterations by Targeting Dysregulated Redox Homeostasis. J Mol Neurosci 73, 403–422 (2023). https://doi.org/10.1007/s12031-023-02127-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-023-02127-w