Abstract
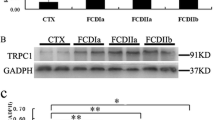
Focal cortical dysplasia (FCD) represents a well-recognized cause of medically intractable epilepsy. Previous studies have indicated that seizures can reduce brain pH and then eliminate seizure discharges. Acid-sensing ion channels (ASICs) are H+-gated cation channels that are widely expressed in the central and peripheral nervous systems. To understand the potential roles of ASIC1a in the epileptogenesis of FCD, we investigated the expression and distribution patterns of ASIC1a in surgical specimens from patients with FCD and age-matched normal cortices (CTX). Decreased ASIC1a messenger RNA (mRNA) and protein expression were detected in FCD compared with CTX. Moreover, the expression of ASIC1a was significantly lower in FCD type II than FCD type I. Immunohistochemistry results indicated that the overall immunoreactivity of the ASIC1a staining was diminished in the dysplastic cortices of FCD compared to the CTX samples. In FCD, ASIC1a immunoreactivity was mainly observed in reactive astrocytes and a minority of malformed cells, including hypertrophic neurons, dysmorphic neurons, and balloon cells. Confocal analysis demonstrated that most malformed cells expressing ASIC1a were co-labeled with neuronal rather than astrocytic markers, indicating a neuronal lineage. In conclusion, the downregulation and altered cellular distribution of ASIC1a in FCD suggest that ASIC1a may potentially contribute to the epileptogenesis of FCD.



Similar content being viewed by others
References
Ali A, Pillai KP, Ahmad FJ, Dua Y, Vohora D (2006) Anticonvulsant effect of amiloride in pentetrazole-induced status epilepticus in mice. Pharmacol Rep 58(2):242–245
Biagini G, Babinski K, Avoli M, Marcinkiewicz M, Seguela P (2001) Regional and subunit-specific downregulation of acid-sensing ion channels in the pilocarpine model of epilepsy. Neurobiol Dis 8(1):45–58
Blumcke I, Thom M, Aronica E et al (2011) The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia 52(1):158–174
Bolshakov KV, Essin KV, Buldakova SL et al (2002) Characterization of acid-sensitive ion channels in freshly isolated rat brain neurons. Neuroscience 110(4):723–730
Engel J Jr (1993) Outcome with respect to epileptic seizures. Surgical treatment of the epilepsies, 2nd edn. Raven, New York, pp 609–621
Friese MA, Craner MJ, Etzensperger R et al (2007) Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med 13(12):1483–1489
Gu L, Liu X, Yang Y, Luo D, Zheng X (2010) ASICs aggravate acidosis-induced injuries during ischemic reperfusion. Neurosci Lett 479(1):63–68
Guo W, Zhang CQ, Shu HF, Yang MH, Yin Q, Yang H (2012) Expression of bone morphogenetic protein-4 in the cortical lesions of focal cortical dysplasia IIb and the tuberous sclerosis complex. J Mol Neurosci 50(1):7–13
Leng TD, Xiong ZG (2013) The pharmacology and therapeutic potential of small molecule inhibitors of acid-sensing ion channels in stroke intervention. Acta Pharmacol Sin 34(1):33–38
Li M, Inoue K, Branigan D et al (2010) Acid-sensing ion channels in acidosis-induced injury of human brain neurons. J Cereb Blood Flow Metab 30(6):1247–1260
Luszczki JJ, Sawicka KM, Kozinska J, Dudra-Jastrzebska M, Czuczwar SJ (2009) Amiloride enhances the anticonvulsant action of various antiepileptic drugs in the mouse maximal electroshock seizure model. J Neural Transm 116(1):57–66
Lv RJ, He JS, Fu YH et al (2011) ASIC1a polymorphism is associated with temporal lobe epilepsy. Epilepsy Res 96(1–2):74–80
N’Gouemo P (2008) Amiloride delays the onset of pilocarpine-induced seizures in rats. Brain Res 1222:230–232
Piao YS, Lu DH, Chen L et al (2010) Neuropathological findings in intractable epilepsy: 435 Chinese cases. Brain Pathol 20(5):902–908
Pignataro G, Simon RP, Xiong ZG (2007) Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain 130(Pt 1):151–158
Roper SN, Eisenschenk S, King MA (1999) Reduced density of parvalbumin- and calbindin D28-immunoreactive neurons in experimental cortical dysplasia. Epilepsy Res 37(1):63–71
Somjen GG (1984) Acidification of interstitial fluid in hippocampal formation caused by seizures and by spreading depression. Brain Res 311(1):186–188
Spreafico R, Battaglia G, Arcelli P et al (1998) Cortical dysplasia: an immunocytochemical study of three patients. Neurology 50(1):27–36
Tolner EA, Hochman DW, Hassinen P et al (2011) Five percent CO(2) is a potent, fast-acting inhalation anticonvulsant. Epilepsia 52(1):104–114
Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M (1997) A proton-gated cation channel involved in acid-sensing. Nature 386(6621):173–177
Wemmie JA, Chen J, Askwith CC et al (2002) The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron 34(3):463–477
Wemmie JA, Coryell MW, Askwith CC et al (2004) Overexpression of acid-sensing ion channel 1a in transgenic mice increases acquired fear-related behavior. Proc Natl Acad Sci U S A 101(10):3621–3626
Xiong ZG, Zhu XM, Chu XP et al (2004) Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 118(6):687–698
Xiong ZG, Pignataro G, Li M, Chang SY, Simon RP (2008) Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr Opin Pharmacol 8(1):25–32
Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ (2004) Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci U S A 101(17):6752–6757
Zhang CQ, Shu HF, Yin Q et al (2012) Expression and cellular distribution of vascular endothelial growth factor-C system in cortical tubers of the tuberous sclerosis complex. Brain Pathol 22(2):205–218
Ziemann AE, Schnizler MK, Albert GW et al (2008) Seizure termination by acidosis depends on ASIC1a. Nat Neurosci 11(7):816–822
Acknowledgments
The investigators would like to thank the technicians, Jin Peng (Central Laboratory of Xiaoqiao Hospital, Third Military Medical University, Chongqing, People’s Republic of China), for her excellent assistance with the laser scanning confocal microscopy. This study was supported by National Natural Science Foundation of China (No. 81271436, No.81370028) and the Natural Science Foundation Project of CQ CSTC (No. cstc2012jjB10019).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
(JPEG 54 kb)
Supplemental Fig. 2
(JPEG 127 kb)
Supplemental Table 1
(DOC 42 kb)
Supplemental Table 2
(DOC 38 kb)
Supplemental Table 3
(DOC 33 kb)
Rights and permissions
About this article
Cite this article
Guo, W., Chen, X., He, JJ. et al. Down-Regulated Expression of Acid-Sensing Ion Channel 1a in Cortical Lesions of Patients with Focal Cortical Dysplasia. J Mol Neurosci 53, 176–182 (2014). https://doi.org/10.1007/s12031-014-0270-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-014-0270-2