Abstract
Purpose
Esophageal cancer is among the leading causes of cancer-related mortality worldwide. Patients presenting with localized and loco-regionally advanced cancer without distant metastases have reasonable survival with multimodality management. Adequate and comprehensive staging is the backbone for proper selection of patients fit for curative treatment. Positron emission tomography (PET) in combination with contrast-enhanced computed tomography (CECT) is utilized as the standard staging modality. Multimodality treatment has been able to achieve evaluable tumor responses including pathological complete response (pCR). It is, therefore, necessary to understand whether the impact of neoadjuvant therapy can be evaluated on imaging, i.e., standardized uptake value (SUV) on PET scan done for response assessment and if this can be correlated with histopathological response and later, with survival. Squamous cell carcinoma (SCC) is more common globally and in the Indian subcontinent; hence, we chose this subgroup to evaluate our hypothesis.
Methods
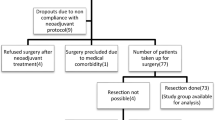
This is a single institution, retrospective study. Out of the 1967 patients who were treated between 2009 and 2019, 1369 (78.54%) patients had SCC. Out of these, 44 received NACTRT, whereas 1325 received NACT followed by curative surgery. The standardized uptake value (SUV) of 18-fluorodeoxyglucose was recorded during pre- and post-neoadjuvant treatment (NAT) using positron emission tomography (PET). The histopathology of the final resection specimen was evaluated using the Mandard tumor regression grade (TRG) criteria with response being graded from 0 to 5 as no residual tumor (NRT), scanty residual tumor (SRT), and residual tumor We attempted to find a cut-off value of the post neoadjuvant SUV of the primary tumor site which correlated with achievement of better histopathological response.
Results
Out of 1325 patients of SCC esophagus who underwent surgery, 943 patients had available data of TRG, and it was categorized into the 0–2 category which had 325 patients (34.5%) and 3–5 category, 618 patients (65.5%). The SUV was taken only from the PET scans done at our institution, so as to achieve a more homogenous cohort, and this was available for 186 patients, 151 from the NACT group and 35 from the NACTRT group. The ROC method was used to find the cut-off for SUV (5.05) in the NACT cohort, which depicted significant difference in the outcome. Out of these, 93 patients who underwent NACT had SUV > 5.05 and 58 had SUV < 5.05. It was found that the subjective and objective histopathological scores correlated at a p value of < 0.0001. Specifically, the majority of cases with SRT tended to be in the 3–5 category of TRG, whereas cases with NRT are predominantly in the 0–2 category. In the ≥ 5.05 category of SUV, there were 76 cases with SRT. In the NACT cohort, the < 5.05 category of SUV, there are 26 cases with SRT and 32 cases with NRT. Among cases with SRT, 74.5% had SUV ≥ 5.05, while 25.5% had SUV < 5.05. Among cases with NRT, 34.7% had SUV ≥ 5.05, while 65.3% had SUV < 5.05 (p value 0.007). No significant association was found in the radio-pathological correlation in the NACTRT group.
Conclusion
Our study confirms the correlation of post neoadjuvant chemotherapy PET SUV with histopathological response, the cut-off of SUV being 5.05 in our cohort. This confirms the predictive value of FDG PET as demonstrated in other studies. Furthermore, its prognostic value with respect to survival has been verified in multiple other studies. With larger scale randomized studies, we may be able to identify the group of patients who have borderline operability anatomically as well as physiologically, where alternative treatment regimens may be indicated to improve outcomes.


Similar content being viewed by others
Data Availability
The dataset generated and analyzed during the current study is available from the corresponding author on reasonable request.
References
Choksi D, et al. Esophageal carcinoma: an epidemiological analysis and study of the time trends over the last 20 years from a single center in India. J Family Med Prim Care. 2020;9(3):1695–9. https://doi.org/10.4103/jfmpc.jfmpc_1111_19.
Smithers BM, et al. Positron emission tomography and pathological evidence of response to neoadjuvant therapy in adenocarcinoma of the esophagus. Dis Esophagus. 2008;21(2):151–8. https://doi.org/10.1111/j.1442-2050.2007.00732.x.
Monjazeb AM, et al. Outcomes of Patients With Esophageal Cancer Staged With [18F]Fluorodeoxyglucose Positron Emission Tomography (FDG-PET): Can Postchemoradiotherapy FDG-PET Predict the Utility of Resection? J Clin Oncol. 2010;28(31):4714–21. https://doi.org/10.1200/JCO.2010.30.7702.
Napier KJ, Scheerer M, Misra S. Esophageal cancer: a review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol. 2014;6(5):112–20. https://doi.org/10.4251/wjgo.v6.i5.112.
Mandard A-M, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73(11):2680–6. https://doi.org/10.1002/1097-0142(19940601)73:11%3c2680::AID-CNCR2820731105%3e3.0.CO;2-C.
Brücher BLDM, et al. Neoadjuvant therapy of esophageal squamous cell carcinoma: response evaluation by positron emission tomography. Ann Surg. 2001;233(3):300–9.
Swisher SG, et al. 2-Fluoro-2-deoxy-D-glucose positron emission tomography imaging is predictive of pathologic response and survival after preoperative chemoradiation in patients with esophageal carcinoma. Cancer. 2004;101(8):1776–85. https://doi.org/10.1002/cncr.20585.
Westerterp M, et al. Monitoring of response to pre-operative chemoradiation in combination with hyperthermia in oesophageal cancer by FDG-PET. Int J Hyperthermia. 2006;22(2):149–60. https://doi.org/10.1080/02656730500513523.
Cerfolio RJ, Bryant AS, Talati AA, Eloubeidi MA, Cerfolio RM, Winokur TS. Change in maximum standardized uptake value on repeat positron emission tomography after chemoradiotherapy in patients with esophageal cancer identifies complete responders. J Thorac Cardiovasc Surg. 2009;137(3):605–9. https://doi.org/10.1016/j.jtcvs.2008.11.016.
Konski AA, et al. Correlation of molecular response as measured by 18-FDG positron emission tomography with outcome after chemoradiotherapy in patients with esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2007;69(2):358–63. https://doi.org/10.1016/j.ijrobp.2007.03.053.
Long NM, Smith CS. Causes and imaging features of false positives and false negatives on 18F-PET/CT in oncologic imaging. Insights Imaging. 2011;2(6):679–98. https://doi.org/10.1007/s13244-010-0062-3.
“PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial - The Lancet Oncology.” [Online]. Available: https://www.thelancet.com/journals/lanonc/article/PIIS1470204507702449/fulltext. Accessed 28 Sep 2023.
Ott K, Herrmann K, Krause B-J, Lordick F. The value of PET imaging in patients with localized gastroesophageal cancer. Gastrointest Cancer Res. 2008;2(6):287–94.
“Randomized phase II study of PET response-adapted combined modality therapy for esophageal cancer: mature results of the CALGB 80803 (alliance) trial - PubMed.” [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/34077237/. Accessed 28 Sep 2023.
McLoughlin JM, et al. Are patients with esophageal cancer who become PET negative after neoadjuvant chemoradiation free of cancer? J Am Coll Surg. 2008;206(5):879–86. https://doi.org/10.1016/j.jamcollsurg.2007.12.027.
Kumar N, et al. Comparison of treatment response assessed by 18F-FDG PET/CT with the histopathological response using tumor regression grading on surgically resected specimen following neoadjuvant chemotherapy in squamous cell carcinoma of esophagus. Nucl Med Commun. 2021;42(8):928–34. https://doi.org/10.1097/MNM.0000000000001413.
Levine EA, et al. Predictive value of 18-fluoro-deoxy-glucose-positron emission tomography (18F-FDG-PET) in the identification of responders to chemoradiation therapy for the treatment of locally advanced esophageal cancer. Ann Surg. 2006;243(4):472–8. https://doi.org/10.1097/01.sla.0000208430.07050.61.
Flamen P, et al. Positron emission tomography for assessment of the response to induction radiochemotherapy in locally advanced oesophageal cancer. Ann Oncol. 2002;13(3):361–8. https://doi.org/10.1093/annonc/mdf081.
“Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment - PubMed.” [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/14990646/. Accessed 28 Sep 2023.
Mantziari S, et al. 18F- FDG PET/CT-derived parameters predict clinical stage and prognosis of esophageal cancer. BMC Med Imaging. 2020;20(1):7. https://doi.org/10.1186/s12880-019-0401-x.
Kroese T1E, et al. Detection of distant interval metastases after neoadjuvant therapy for esophageal cancer with 18F-FDG PET(/CT): a systematic review and meta-analysis. Dis Esophagus. 2018;31(12). https://doi.org/10.1093/dote/doy055.
Motoori M, et al. Early response to neoadjuvant chemotherapy in advanced esophageal cancer evaluated by computed tomography predicts the utility of a second cycle of chemotherapy. Mole Clin Oncol. 2013;1(3):521–6. https://doi.org/10.3892/mco.2013.89.
Hermus M, et al. Patient preferences for active surveillance vs standard surgery after neoadjuvant chemoradiotherapy in oesophageal cancer treatment: the NOSANO-study. Int J Cancer. 2023;152(6):1183–90. https://doi.org/10.1002/ijc.34327.
Hermus M, et al. Esophageal cancer patients’ need for information and support in making a treatment decision between standard surgery and active surveillance. Cancer Med. 2023;12(16):17266–72. https://doi.org/10.1002/cam4.6308.
van der Zijden CJ, et al. A prospective cohort study on active surveillance after neoadjuvant chemoradiotherapy for esophageal cancer: protocol of Surgery As Needed for Oesophageal cancer-2. BMC Cancer. 2023;23(1):327. https://doi.org/10.1186/s12885-023-10747-z.
Bhargava P, Rahman S, Wendt J. Atlas of confounding factors in head and neck PET/CT imaging. Clin Nucl Med. 2011;36(5):e20-29. https://doi.org/10.1097/RLU.0b013e318212c872.
Benveniste MF, et al. Recognizing radiation therapy–related complications in the chest. Radiographics. 2019;39(2):344–66. https://doi.org/10.1148/rg.2019180061.
Funding
The authors confirm that no money, grants, or other forms of support were provided during this study.
Author information
Authors and Affiliations
Contributions
S.J. conceptualized the paper. A.K. and S.J. wrote the main manuscript. A.K. did the data collection. A.P. did the statistical analysis. All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This is an observational study. The TMH Ethics committee has confirmed that no ethics approval is required.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaderi, A.A., Sabita, J., Tiwari, V.K. et al. Treatment Response to Neoadjuvant Therapy in Squamous Esophageal Cancer—Correlation Between Metabolic Response and Histopathology. J Gastrointest Canc (2024). https://doi.org/10.1007/s12029-024-01013-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s12029-024-01013-x