Abstract
Objective
Hepatocellular cancer (HCC) is an aggressive tumor with an increasing incidence in recent years. Life expectancy is limited, especially due to limited effective treatments and tumor biology. In this study, we aimed to examine the effect of neutrophil–lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), prognostic nutritional index (PNI) parameters of treatment efficacy of patients using sorafenib in primary systemic therapy, progression-free survival (PFS), and overall survival (OS).
Materials and Methods
In this study, we retrospectively analyzed 78 patients who used sorafenib as a first-line systemic treatment. NLR, PLR, and PNI values were calculated with the existing formulas. Cut-off values for these markers were determined by performing ROC curve analysis. These values were determined respectively as 2.88, 111.05, and 38.25. Patients were divided into two groups according to this threshold value. OS and PFS values were calculated using a Cox proportional risk model. The effects of markers on OS and PFS were examined based on the cut-off value.
Results
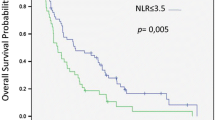
The mean PFS was 7.1 (range 1–46) months, and the mean OS was 14.1 (range 1.5–94) months. The pre-treatment decreased NLR (< 2.88) value was prognostic for higher PFS and OS rates. These values were determined respectively as 9.23 ± 1.79 and 3.45 ± 0.32 months for PFS and 21.17 ± 4.53 and 5.32 ± 0.53 months for OS. Pre-treatment decreased PLR (< 111.05) was found to be a positively significant prognostic value for both survival. These values were determined respectively as 7.37 ± 1.43 months and 3.16 ± 0.47 months for PFS and 21.12 ± 5.52 months and 6.16 ± 0.87 months for OS. And also, low PNI (< 38.25) value was prognostic for lower PFS and rates. These values were determined respectively as 7.47 ± 0.59 months and 3.25 ± 0.21 months for PFS and 16.36 ± 4.37 months and 5.15 ± 0.42 for OS. All three parameters were found to be statistically significant (p < 0.05) for both OS and PFS as independent prognostic markers.
Conclusion
Today, as the standard first-line treatment of HCC has shifted to combinations with immunotherapy (IO), IO transportation is not possible in most countries of the world. However, there are also patients who achieve great survival with only sorafenib. The important point is to identify the biomarkers that predict which patient will benefit better from which treatment. With the markers in our study and a scoring system that can be obtained with these markers, it can be evaluated which patient will be given IO combination and which patient will be given only TKI treatment. We think that such a scoring system can be used to identify suitable patients, especially in countries where, for financial reasons, not every patient can access Immunotherapy. The advantage of these tests is that they are inexpensive, easily calculable and standardized.
Trial Registration
Number and date of registration: 2021/2088, 01–06-2021, retrospectively registered.







Similar content being viewed by others
Data Availability
There is data availability. We can provide if requested.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. https://doi.org/10.3322/caac.21262.
Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. https://doi.org/10.1056/nejmoa0708857.
Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73. https://doi.org/10.1016/S0140-6736(18)30207-1.
Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–905. https://doi.org/10.1056/nejmoa1915745.
Ringelhan M, Pfister D, O’Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma review-article. Nat Immunol. 2018;19(3):222–32. https://doi.org/10.1038/s41590-018-0044-z.
Guthrie GJK, Charles KA, Roxburgh CSD, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–30. https://doi.org/10.1016/j.critrevonc.2013.03.010.
Zhou X, Du Y, Huang Z, et al. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One. 2014;9(6). https://doi.org/10.1371/journal.pone.0101119
Li S, Guo JH, Lu J, Wang C, Wang H. Prognostic value of preoperative prognostic nutritional index and body mass index combination in patients with unresectable hepatocellular carcinoma after transarterial chemoembolization. Cancer Manag Res. 2021;13:1637–50. https://doi.org/10.2147/CMAR.S290983.
Zheng J, Cai J, Li H, et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as prognostic predictors for hepatocellular carcinoma patients with various treatments: a meta-analysis and systematic review. Cell Physiol Biochem. 2017;44(3):967–81. https://doi.org/10.1159/000485396.
Hatanaka T, Kakizaki S, Uehara D, et al. Impact of the prognostic nutritional index on the survival of Japanese patients with hepatocellular carcinoma treated with sorafenib: a multicenter retrospective study. Intern Med. 2019;58(13):1835–44. https://doi.org/10.2169/internalmedicine.1594-18.
Caputo F, Dadduzio V, Tovoli F, et al. The role of PNI to predict survival in advanced hepatocellular carcinoma treated with Sorafenib. PLoS One. 2020;15(5):1–13. https://doi.org/10.1371/journal.pone.0232449.
Lin WF, Zhong MF, Zhang YR, et al. Prognostic role of platelet-to-lymphocyte ratio in hepatocellular carcinoma with different BCLC Stages: a systematic review and meta-analysis. Gastroenterology Res Pract. 2018;2018. https://doi.org/10.1155/2018/5670949
Suner A, Carr BI. Platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios predict tumor size and survival in HCC patients: retrospective study. Ann Med Surg. 2020;58(August):167–71. https://doi.org/10.1016/j.amsu.2020.08.042.
Ren Z, Fan J, Xu J et al. No title. Ann Oncol. 2020;31(Suppl_6):S1287–S318.
Heng DYC, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–9. https://doi.org/10.1200/JCO.2008.21.4809.
Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–90. https://doi.org/10.1056/nejmoa1712126.
Author information
Authors and Affiliations
Contributions
Conception: constructing an idea or hypothesis for research and/or manuscript, HH; design: planning methodology to reach the conclusion, HH; supervision: organizing and supervising the course of the project or the article and taking the responsibility, AG., HH; fundings: providing personnel, environmental and financial support and tools and instruments that are vital for the project. There is no funding; materials: biological materials, reagents and referred patients, AG; data collection and/or processing: taking responsibility in execution of the experiments, patient follow-up, data management and reporting, AG; analysis and/or interpretation: taking responsibility in logical interpretation and presentation of the results, AG., HH; literature review: taking responsibility in this necessary function, AG; writer: taking responsibility in the construction of the whole or body of the manuscript, AG; critical review: reviewing the article before submission not only for spelling and grammar but also for its intellectual content, HH.
Corresponding author
Ethics declarations
Ethics Approval
Our retrospectively planned study complies with the Declaration of Helsinki. Ethics committee approval was obtained from our institution.
Consent to Participate
We confirm participation.
Consent for Publication
In case the article is accepted, we declare that we have transferred all our rights to the journal to publish the article.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gulmez, A., Harputluoglu, H. Advanced Hepatocellular Cancer Treated with Sorafenib and Novel Inflammatory Markers. J Gastrointest Canc 54, 11–19 (2023). https://doi.org/10.1007/s12029-021-00789-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-021-00789-6