Abstract
Background
Serum neutrophil–lymphocyte ratio (NLR) is a surrogate marker for the inflammatory response after intracerebral hemorrhage (ICH) and is associated with perihematomal edema and long-term functional outcomes. Whether NLR is associated with short-term ICH complications is poorly understood. We hypothesized that NLR is associated with 30-day infection and thrombotic events after ICH.
Methods
We performed a post hoc exploratory analysis of the Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage III trial. The study exposure was the serum NLR obtained at baseline and on days 3 and 5. The coprimary outcomes, ascertained at 30 days, were any infection and a thrombotic event, defined as composite of cerebral infarction, myocardial infarction, or venous thromboembolism; both infection and thrombotic event were determined through adjudicated adverse event reporting. Binary logistic regression was used to study the relationship between NLR and outcomes, after adjustment for demographics, ICH severity and location, and treatment randomization.
Results
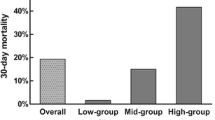
Among the 500 patients enrolled in the Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage III trial, we included 303 (60.6%) without missing data on differential white blood cell counts at baseline. There were no differences in demographics, comorbidities, or ICH severity between patients with and without data on NLR. In adjusted logistic regression models, NLR ascertained at baseline (odds ratio [OR] 1.03; 95% confidence interval [CI] 1.01–1.07, p = 0.03) and NLR ascertained at day 3 were associated with infection (OR 1.15; 95% CI 1.05–1.20, p = 0.001) but not with thrombotic events. Conversely, NLR at day 5 was associated with thrombotic events (OR 1.07, 95% CI 1.01–1.13, p = 0.03) but not with infection (OR 1.13; 95% CI 0.76–1.70, p = 0.56). NLR at baseline was not associated with either outcome.
Conclusions
Serum NLR ascertained at baseline and on day 3 after randomization was associated with 30-day infection, whereas NLR obtained on day 5 was associated with thrombotic events after ICH, suggesting that NLR could be a potential early biomarker for ICH-related complications.


Similar content being viewed by others
References
van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9(2):167–76.
Li L, Murthy SB. Cardiovascular events after intracerebral hemorrhage. Stroke. 2022;53(7):2131–41.
Shah VA, Thompson RE, Yenokyan G, et al. One-year outcome trajectories and factors associated with functional recovery among survivors of intracerebral and intraventricular hemorrhage with initial severe disability. JAMA Neurol. 2022;79(9):856–68.
Parasram M, Parikh NS, Merkler AE, et al. Risk of mortality after an arterial ischemic event among intracerebral hemorrhage survivors. Neurohospitalist. 2022;12(1):19–23.
Investigators CIT. Venous thromboembolism after intraventricular hemorrhage: results from the CLEAR III trial. Neurosurgery. 2019;84(3):709–16.
Ziai WC. Hematology and inflammatory signaling of intracerebral hemorrhage. Stroke. 2013;44(6 Suppl 1):S74–8.
Shi K, Wood K, Shi FD, Wang X, Liu Q. Stroke-induced immunosuppression and poststroke infection. Stroke Vasc Neurol. 2018;3(1):34–41.
Kamel H, Iadecola C. Brain–immune interactions and ischemic stroke: clinical implications. Arch Neurol. 2012;69(5):576–81.
Wang F, Hu S, Ding Y, et al. Neutrophil-to-lymphocyte ratio and 30-day mortality in patients with acute intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2016;25(1):182–7.
Sun Y, You S, Zhong C, et al. Neutrophil to lymphocyte ratio and the hematoma volume and stroke severity in acute intracerebral hemorrhage patients. Am J Emerg Med. 2017;35(3):429–33.
Qin J, Li Z, Gong G, et al. Early increased neutrophil-to-lymphocyte ratio is associated with poor 3-month outcomes in spontaneous intracerebral hemorrhage. PLoS ONE. 2019;14(2):e0211833.
Shaafi S, Bonakdari E, Sadeghpour Y, Nejadghaderi SA. Correlation between red blood cell distribution width, neutrophil to lymphocyte ratio, and neutrophil to platelet ratio with 3-month prognosis of patients with intracerebral hemorrhage: a retrospective study. BMC Neurol. 2022;22(1):191.
Giede-Jeppe A, Madzar D, Sembill JA, et al. Increased neutrophil-to-lymphocyte ratio is associated with unfavorable functional outcome in acute ischemic stroke. Neurocrit Care. 2020;33(1):97–104.
Chang JJ, Dowlati E, Triano M, et al. Admission neutrophil to lymphocyte ratio for predicting outcome in subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2021;30(9):105936.
Gusdon AM, Gialdini G, Kone G, et al. Neutrophil–lymphocyte ratio and perihematomal edema growth in intracerebral hemorrhage. Stroke. 2017;48(9):2589–92.
Hanley DF, Lane K, McBee N, et al. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet. 2017;389(10069):603–11.
von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–7.
Ziai WC, Tuhrim S, Lane K, et al. A multicenter, randomized, double-blinded, placebo-controlled phase III study of clot lysis evaluation of accelerated resolution of intraventricular hemorrhage (CLEAR III). Int J Stroke. 2014;9(4):536–42.
Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31(4):1250–6.
Fam MD, Zeineddine HA, Eliyas JK, et al. CSF inflammatory response after intraventricular hemorrhage. Neurology. 2017;89(15):1553–60.
Rivera-Lara L, Murthy SB, Nekoovaght-Tak S, et al. Influence of bleeding pattern on ischemic lesions after spontaneous hypertensive intracerebral hemorrhage with intraventricular hemorrhage. Neurocrit Care. 2018;29(2):180–8.
Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–35.
Gusdon AM, Thompson CB, Quirk K, et al. CSF and serum inflammatory response and association with outcomes in spontaneous intracerebral hemorrhage with intraventricular extension: an analysis of the CLEAR-III Trial. J Neuroinflammation. 2021;18(1):179.
Li X, Chen G. CNS-peripheral immune interactions in hemorrhagic stroke. J Cereb Blood Flow Metab. 2023;43(2):185–97.
Alimohammadi E, Bagheri SR, Mardanpour P, Moradi F, Arjmandnia F, Esmaeili N. Baseline neutrophil-lymphocyte ratio can be associated with hematoma expansion in patients with intracerebral hemorrhage: a retrospective observational study. BMC Neurosci. 2022;23(1):18.
Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Neutrophil-to-lymphocyte ratio predicts the outcome of acute intracerebral hemorrhage. Stroke. 2016;47(6):1654–7.
Huang Z, Fu Z, Huang W, Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: a meta-analysis. Am J Emerg Med. 2020;38(3):641–7.
Nam KW, Kim TJ, Lee JS, et al. High neutrophil-to-lymphocyte ratio predicts stroke-associated pneumonia. Stroke. 2018;49(8):1886–92.
Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133(9):906–18.
Maugeri N, Brambilla M, Camera M, et al. Human polymorphonuclear leukocytes produce and express functional tissue factor upon stimulation. J Thromb Haemost. 2006;4(6):1323–30.
Mocsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013;210(7):1283–99.
Massberg S, Grahl L, von Bruehl ML, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16(8):887–96.
Gould TJ, Vu TT, Swystun LL, et al. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol. 2014;34(9):1977–84.
Hannawi Y, Hannawi B, Rao CP, Suarez JI, Bershad EM. Stroke-associated pneumonia: major advances and obstacles. Cerebrovasc Dis. 2013;35(5):430–43.
Funding
This study was supported by the National Institutes of Health through the following grants: National Institutes of Health 5U01NS062851 (Drs. Hanley, Awad, and Ziai) and K23NS105948 (Dr. Murthy). The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Dr. SBM had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: SK, SBM. Acquisition, analysis, or interpretation of data: SBM, CZ. Drafting of the manuscript: SK, WCZ, SBM. Critical revision of the manuscript for important intellectual content: SK, CZ, AMG, SO, AEM, IA, DFH, HK, WCZ, SBM. Statistical analysis: SBM. Administrative, technical, or material support: SBM. Study supervision: WCZ, SBM. The final manuscript was approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Merkler reports personal fees for medicolegal consulting in stroke and neurological disorders. Dr. Awad is supported by the National Institutes of Health (NIH) (1U01NS080824 and U01NS106513). Dr. Hanley is supported by the NIH (U01NS080824 and U24TR001609), and reports personal fees from Op2Lysis, personal fees from BrainScope and Neurotrope, and nonfinancial support from Genentech outside the submitted work. Dr. Kamel has received grant support from the NIH, serves as Co-principal investigator (co-PI) for the NIH-funded AtRial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke (ARCADIA) trial (National Institute of Neurological Disorders and Stroke U01NS095869) which receives in-kind study drug from the Bristol Myers Squibb BMS-Pfizer Alliance for Eliquis and ancillary study support from Roche Diagnostics, serves as Deputy Editor for JAMA Neurology, serves as a steering committee member of Medtronic’s Stroke atrial fibrillation (AF) trial (uncompensated), serves on an endpoint adjudication committee for a trial of empagliflozin for Boehringer-Ingelheim, and has served on an advisory board for Roivant Sciences related to Factor XI inhibition. Dr. Ziai is supported by the NIH (1U01NS080824, R01NS102583, and U01NS106513), and receives consulting fees from C.R. Bard, Inc. outside of the area of work commented on here. Dr. Murthy is supported by the NIH (K23NS105948) and has received personal fees for medicolegal consulting on neurological disorders. The other authors have nothing to disclose.
Ethical Approval/Informed Consent
The article adheres to ethical guidelines and indicates ethical approvals (institutional review board). The study was approved by the Weill Cornell Medicine Institutional Review Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaleem, S., Zhang, C., Gusdon, A.M. et al. Association Between Neutrophil–Lymphocyte Ratio and 30-Day Infection and Thrombotic Outcomes After Intraventricular Hemorrhage: A CLEAR III Analysis. Neurocrit Care 40, 529–537 (2024). https://doi.org/10.1007/s12028-023-01774-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-023-01774-6