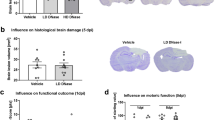
Abstract
Background
Understanding the secondary damage mechanisms of traumatic brain injury (TBI) is essential for developing new therapeutic approaches. Neuroinflammation has a pivotal role in secondary brain injury after TBI. Activation of NLRP3 inflammasome complexes results in the secretion of proinflammatory mediators and, in addition, later in the response, microglial activation and migration of the peripheral immune cells into the injured brain are observed. Therefore, these components involved in the inflammatory process are becoming a new treatment target in TBI. Dexmedetomidine (Dex) is an effective drug, widely used over the past few years in neurocritical care units and during surgical operations for sedation and analgesia, and has anti-inflammatory effects, which are shown in in vivo studies. The aim of this original research is to discuss the anti-inflammatory effects of different Dex doses over time in TBI.
Methods
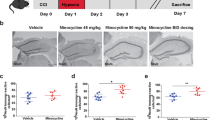
Brain injury was performed by using a weight-drop model. Half an hour after the trauma, intraperitoneal saline was injected into the control groups and 40 and 200 μg/kg of Dex were given to the drug groups. Neurological evaluations were performed with the modified Neurological Severity Score before being killed. Then, the mice were killed on the first or the third day after TBI and histopathologic (hematoxylin–eosin) and immunofluorescent (Iba1, NLRP3, interleukin-1β, and CD3) findings of the brain tissues were examined. Nonparametric data were analyzed by using the Kruskal–Wallis test for multiple comparisons, and the Mann–Whitney U-test was done for comparing two groups. The results are presented as mean ± standard error of mean.
Results
The results showed that low doses of Dex suppress NLRP3 and interleukin-1β in both terms. Additionally, high doses of Dex cause a remarkable decrease in the migration and motility of microglial cells and T cells in the late phase following TBI. Interestingly, the immune cells were influenced by only high-dose Dex in the late phase of TBI and it also improves neurologic outcome in the same period.
Conclusions
In the mice head trauma model, different doses of Dex attenuate neuroinflammation by suppressing distinct components of the neuroinflammatory process in a different timecourse that contributes to neurologic recovery. These results suggest that Dex may be an appropriate choice for sedation and analgesia in patients with TBI.







Similar content being viewed by others
Availability of Data and Materials
The data supporting the conclusions of this article are available from the corresponding author upon reasonable request.
References
Menon DK, Schwab K, Wright DW, Maas AI, Demographics, Clinical Assessment Working Group of the I, et al. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. 2010;91(11):1637–40.
Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7(8):728–41.
Andelic N. The epidemiology of traumatic brain injury. The Lancet Neurology. 2013;12(1):28–9.
McKee AC, Daneshvar DH. The neuropathology of traumatic brain injury. Handb Clin Neurol. 2015;127:45–66.
Chiu CC, Liao YE, Yang LY, Wang JY, Tweedie D, Karnati HK, et al. Neuroinflammation in animal models of traumatic brain injury. J Neurosci Methods. 2016;272:38–49.
Ghajar J. Traumatic brain injury. The Lancet. 2000;356(9233):923–9.
Werner C, Engelhard K. Pathophysiology of traumatic brain injury. BJA Br J Anaesth. 2007;99(1):4–9.
Graham DI, McIntosh TK, Maxwell WL, Nicoll JA. Recent advances in neurotrauma. J Neuropathol Exp Neurol. 2000;59(8):641–51.
Song L, Pei L, Yao S, Wu Y, Shang Y. NLRP3 Inflammasome in Neurological Diseases, from Functions to Therapies. Front Cell Neurosci. 2017;11:63.
Mortimer JA, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, Heyman A, et al. Head trauma as a risk factor for Alzheimer’s disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20(Suppl 2):S28-35.
Webster SJ, Van Eldik LJ, Watterson DM, Bachstetter AD. Closed head injury in an age-related Alzheimer mouse model leads to an altered neuroinflammatory response and persistent cognitive impairment. J Neurosci. 2015;35(16):6554–69.
Harnod D, Harnod T, Lin CL, Shen WC, Kao CH. Increased risks of suicide attempt and suicidal drug overdose following admission for head injury in patients with depression. Int J Environ Res Public Health. 2019;16(19):3524.
Holsinger T, Steffens DC, Phillips C, Helms MJ, Havlik RJ, Breitner JC, et al. Head injury in early adulthood and the lifetime risk of depression. Arch Gen Psychiatry. 2002;59(1):17–22.
Mas F, Prichep LS, Alper K. Treatment resistant depression in a case of minor head injury: an electrophysiological hypothesis. Clin Electroencephalogr. 1993;24(3):118–22.
Salmond CH, Menon DK, Chatfield DA, Pickard JD, Sahakian BJ. Cognitive reserve as a resilience factor against depression after moderate/severe head injury. J Neurotrauma. 2006;23(7):1049–58.
Weiner MW, Harvey D, Hayes J, Landau SM, Aisen PS, Petersen RC, et al. Effects of traumatic brain injury and posttraumatic stress disorder on development of Alzheimer’s disease in Vietnam Veterans using the Alzheimer’s disease neuroimaging initiative: preliminary report. Alzheimers Dement (N Y). 2017;3(2):177–88.
Chase A. Parkinson disease: traumatic brain injury increases the risk of Parkinson disease. Nat Rev Neurol. 2015;11(4):184.
Gardner RC, Burke JF, Nettiksimmons J, Goldman S, Tanner CM, Yaffe K. Traumatic brain injury in later life increases risk for Parkinson disease. Ann Neurol. 2015;77(6):987–95.
Kiernan PT, Montenigro PH, Solomon TM, McKee AC. Chronic traumatic encephalopathy: a neurodegenerative consequence of repetitive traumatic brain injury. Semin Neurol. 2015;35(1):20–8.
Filiano AJ, Gadani SP, Kipnis J. How and why do T cells and their derived cytokines affect the injured and healthy brain? Nat Rev Neurosci. 2017;18(6):375–84.
Freeman LC, Ting JP. The pathogenic role of the inflammasome in neurodegenerative diseases. J Neurochem. 2016;136(Suppl 1):29–38.
Adamczak S, Dale G, de Rivero Vaccari JP, Bullock MR, Dietrich WD, Keane RW. Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: clinical article. J Neurosurg. 2012;117(6):1119–25.
Donat CK, Scott G, Gentleman SM, Sastre M. Microglial activation in traumatic brain injury. Front Aging Neurosci. 2017;9:208.
Smith C, Gentleman SM, Leclercq PD, Murray LS, Griffin WS, Graham DI, et al. The neuroinflammatory response in humans after traumatic brain injury. Neuropathol Appl Neurobiol. 2013;39(6):654–66.
Galgano M, Toshkezi G, Qiu X, Russell T, Chin L, Zhao LR. Traumatic brain injury: current treatment strategies and future endeavors. Cell Transplant. 2017;26(7):1118–30.
Abou El Fadl MH, O’Phelan KH. Management of traumatic brain injury: an update. Neurosurg Clin N Am. 2018;29(2):213–21.
Jain KK. Neuroprotection in traumatic brain injury. Drug Discov Today. 2008;13(23–24):1082–9.
Pearn ML, Niesman IR, Egawa J, Sawada A, Almenar-Queralt A, Shah SB, et al. Pathophysiology associated with traumatic brain injury: current treatments and potential novel therapeutics. Cell Mol Neurobiol. 2017;37(4):571–85.
Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015;72(3):355–62.
Wang L, Liu H, Zhang L, Wang G, Zhang M, Yu Y. Neuroprotection of dexmedetomidine against cerebral ischemia-reperfusion injury in rats: involved in inhibition of NF-kappaB and inflammation response. Biomol Ther (Seoul). 2017;25(4):383–9.
Luo C, Ouyang MW, Fang YY, Li SJ, Zhou Q, Fan J, et al. Dexmedetomidine protects mouse brain from ischemia-reperfusion injury via inhibiting neuronal autophagy through up-regulating HIF-1alpha. Front Cell Neurosci. 2017;11:197.
Bell MT, Agoston VA, Freeman KA, Puskas F, Herson PS, Mares J, et al. Interruption of spinal cord microglial signaling by alpha-2 agonist dexmedetomidine in a murine model of delayed paraplegia. J Vasc Surg. 2014;59(4):1090–7.
Ma J, Zhang XL, Wang CY, Lin Z, Tao JR, Liu HC. Dexmedetomidine alleviates the spinal cord ischemia-reperfusion injury through blocking mast cell degranulation. Int J Clin Exp Med. 2015;8(9):14741–9.
Rong H, Zhao Z, Feng J, Lei Y, Wu H, Sun R, et al. The effects of dexmedetomidine pretreatment on the pro- and anti-inflammation systems after spinal cord injury in rats. Brain Behav Immun. 2017;64:195–207.
Liu Z, Wang Y, Wang Y, Ning Q, Zhang Y, Gong C, et al. Dexmedetomidine attenuates inflammatory reaction in the lung tissues of septic mice by activating cholinergic anti-inflammatory pathway. Int Immunopharmacol. 2016;35:210–6.
Liu W, Yu W, Weng Y, Wang Y, Sheng M. Dexmedetomidine ameliorates the inflammatory immune response in rats with acute kidney damage. Exp Ther Med. 2017;14(4):3602–8.
Sun Y, Jiang C, Jiang J, Qiu L. Dexmedetomidine protects mice against myocardium ischaemic/reperfusion injury by activating an AMPK/PI3K/Akt/eNOS pathway. Clin Exp Pharmacol Physiol. 2017;44(9):946–53.
Chen Z, Ding T, Ma CG. Dexmedetomidine (DEX) protects against hepatic ischemia/reperfusion (I/R) injury by suppressing inflammation and oxidative stress in NLRC5 deficient mice. Biochem Biophys Res Commun. 2017;493(2):1143–50.
Li Y, Pan Y, Gao L, Lu G, Zhang J, Xie X, et al. Dexmedetomidine attenuates pancreatic injury and inflammatory response in mice with pancreatitis by possible reduction of NLRP3 activation and up-regulation of NET expression. Biochem Biophys Res Commun. 2018;495(4):2439–47.
Cheng F, Yan FF, Liu YP, Cong Y, Sun KF, He XM. Dexmedetomidine inhibits the NF-kappaB pathway and NLRP3 inflammasome to attenuate papain-induced osteoarthritis in rats. Pharm Biol. 2019;57(1):649–59.
Jiang WW, Wang QH, Liao YJ, Peng P, Xu M, Yin LX. Effects of dexmedetomidine on TNF-alpha and interleukin-2 in serum of rats with severe craniocerebral injury. BMC Anesthesiol. 2017;17(1):130.
Schoeler M, Loetscher PD, Rossaint R, Fahlenkamp AV, Eberhardt G, Rex S, et al. Dexmedetomidine is neuroprotective in an in vitro model for traumatic brain injury. BMC Neurol. 2012;12:20.
Humble SS, Wilson LD, Leath TC, Marshall MD, Sun DZ, Pandharipande PP, et al. ICU sedation with dexmedetomidine after severe traumatic brain injury. Brain Inj. 2016;30(10):1266–70.
Ma D, Rajakumaraswamy N, Maze M. alpha2-Adrenoceptor agonists: shedding light on neuroprotection? Br Med Bull. 2004;71:77–92.
Shen M, Wang S, Wen X, Han XR, Wang YJ, Zhou XM, et al. Dexmedetomidine exerts neuroprotective effect via the activation of the PI3K/Akt/mTOR signaling pathway in rats with traumatic brain injury. Biomed Pharmacother. 2017;95:885–93.
Irrera N, Russo M, Pallio G, Bitto A, Mannino F, Minutoli L, et al. The role of NLRP3 inflammasome in the pathogenesis of traumatic brain injury. Int J Mol Sci. 2020;21(17):6204.
Gentleman D. Causes and effects of systemic complications among severely head injured patients transferred to a neurosurgical unit. Int Surg. 1992;77(4):297–302.
Wu J, Vogel T, Gao X, Lin B, Kulwin C, Chen J. Neuroprotective effect of dexmedetomidine in a murine model of traumatic brain injury. Sci Rep. 2018;8(1):4935.
Dahmani S, Paris A, Jannier V, Hein L, Rouelle D, Scholz J, et al. Dexmedetomidine increases hippocampal phosphorylated extracellular signal-regulated protein kinase 1 and 2 content by an alpha 2-adrenoceptor-independent mechanism: evidence for the involvement of imidazoline I1 receptors. Anesthesiology. 2008;108(3):457–66.
Zhu YM, Wang CC, Chen L, Qian LB, Ma LL, Yu J, et al. Both PI3K/Akt and ERK1/2 pathways participate in the protection by dexmedetomidine against transient focal cerebral ischemia/reperfusion injury in rats. Brain Res. 2013;1494:1–8.
Talke P, Bickler PE. Effects of dexmedetomidine on hypoxia-evoked glutamate release and glutamate receptor activity in hippocampal slices. Anesthesiology. 1996;85(3):551–7.
Wang D, Xu X, Wu YG, Lyu L, Zhou ZW, Zhang JN. Dexmedetomidine attenuates traumatic brain injury: action pathway and mechanisms. Neural Regen Res. 2018;13(5):819–26.
Riquelme JA, Westermeier F, Hall AR, Vicencio JM, Pedrozo Z, Ibacache M, et al. Dexmedetomidine protects the heart against ischemia-reperfusion injury by an endothelial eNOS/NO dependent mechanism. Pharmacol Res. 2016;103:318–27.
Liu Z, Wang Y, Ning Q, Gong C, Zhang Y, Zhang L, et al. The role of spleen in the treatment of experimental lipopolysaccharide-induced sepsis with dexmedetomidine. SpringerPlus. 2015;4:800.
Akpinar O, Naziroglu M, Akpinar H. Different doses of dexmedetomidine reduce plasma cytokine production, brain oxidative injury, PARP and caspase expression levels but increase liver oxidative toxicity in cerebral ischemia-induced rats. Brain Res Bull. 2017;130:1–9.
Si Y, Zhang Y, Han L, Chen L, Xu Y, Sun F, et al. Dexmedetomidine acts via the JAK2/STAT3 pathway to attenuate isoflurane-induced neurocognitive deficits in senile mice. PLoS ONE. 2016;11(10):e0164763.
Yeh CH, Hsieh LP, Lin MC, Wei TS, Lin HC, Chang CC, et al. Dexmedetomidine reduces lipopolysaccharide induced neuroinflammation, sickness behavior, and anhedonia. PLoS ONE. 2018;13(1):e0191070.
Zhu YJ, Peng K, Meng XW, Ji FH. Attenuation of neuroinflammation by dexmedetomidine is associated with activation of a cholinergic anti-inflammatory pathway in a rat tibial fracture model. Brain Res. 2016;1644:1–8.
Kutanis D, Erturk E, Besir A, Demirci Y, Kayir S, Akdogan A, et al. Dexmedetomidine acts as an oxidative damage prophylactic in rats exposed to ionizing radiation. J Clin Anesth. 2016;34:577–85.
Bye N, Habgood MD, Callaway JK, Malakooti N, Potter A, Kossmann T, et al. Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp Neurol. 2007;204(1):220–33.
Madathil SK, Wilfred BS, Urankar SE, Yang W, Leung LY, Gilsdorf JS, et al. Early microglial activation following closed-head concussive injury is dominated by pro-inflammatory M-1 type. Front Neurol. 2018;9:964.
Carbonell WS, Murase S, Horwitz AF, Mandell JW. Migration of perilesional microglia after focal brain injury and modulation by CC chemokine receptor 5: an in situ time-lapse confocal imaging study. J Neurosci. 2005;25(30):7040–7.
Liu HD, Li W, Chen ZR, Hu YC, Zhang DD, Shen W, et al. Expression of the NLRP3 inflammasome in cerebral cortex after traumatic brain injury in a rat model. Neurochem Res. 2013;38(10):2072–83.
O’Brien WT, Pham L, Symons GF, Monif M, Shultz SR, McDonald SJ. The NLRP3 inflammasome in traumatic brain injury: potential as a biomarker and therapeutic target. J Neuroinflammation. 2020;17(1):104.
Fann DY, Lee SY, Manzanero S, Tang SC, Gelderblom M, Chunduri P, et al. Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis. 2013;4:e790.
Ma Q, Chen S, Hu Q, Feng H, Zhang JH, Tang J. NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann Neurol. 2014;75(2):209–19.
Diaz MF, Horton PD, Kumar A, Livingston M, Mohammadalipour A, Xue H, et al. Injury intensifies T cell mediated graft-versus-host disease in a humanized model of traumatic brain injury. Sci Rep. 2020;10(1):10729.
Soares HD, Hicks RR, Smith D, McIntosh TK. Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J Neurosci. 1995;15(12):8223–33.
Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–75.
Carlos TM, Clark RS, Franicola-Higgins D, Schiding JK, Kochanek PM. Expression of endothelial adhesion molecules and recruitment of neutrophils after traumatic brain injury in rats. J Leukoc Biol. 1997;61(3):279–85.
Schwarzmaier SM, Plesnila N. Contributions of the immune system to the pathophysiology of traumatic brain injury—evidence by intravital microscopy. Front Cell Neurosci. 2014;8:358.
Kramer TJ, Hack N, Bruhl TJ, Menzel L, Hummel R, Griemert EV, et al. Depletion of regulatory T cells increases T cell brain infiltration, reactive astrogliosis, and interferon-gamma gene expression in acute experimental traumatic brain injury. J Neuroinflammation. 2019;16(1):163.
Acknowledgements
We would like to thank Mesut Fırat for his technical support.
Funding
This study was supported by Hacettepe University Scientific Research Projects Coordination Unit (Project Number: THD-2017-16630), Ankara, Turkey.
Author information
Authors and Affiliations
Contributions
Conception and design: MMA, DK, CCA, FS, and SU. Project administration: MMA, DK and CCA. Acquisition of data: DK and CCA. Analysis and interpretation of data: DK and CCA. Drafting the article: DK and CCA. Critically revising the article: MMA, DK, CCA and FS. Statistical analysis: CCA. Study supervision: MMA. DK and CCA contributed equally. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval/Animal Rights
All experimental procedures involving animals were approved by the Animal Experimentations Local Ethics Board of Hacettepe University (No. 2017/67-01), Ankara, Turkey. All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karakaya, D., Cakir-Aktas, C., Uzun, S. et al. Tailored Therapeutic Doses of Dexmedetomidine in Evolving Neuroinflammation after Traumatic Brain Injury. Neurocrit Care 36, 802–814 (2022). https://doi.org/10.1007/s12028-021-01381-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01381-3